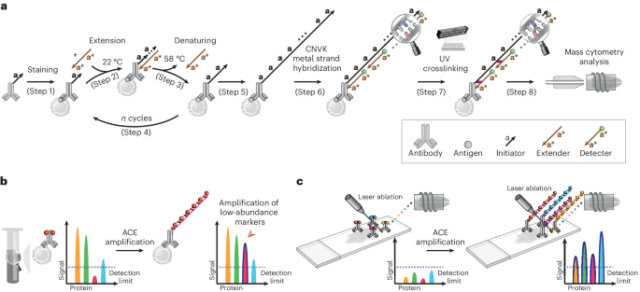
Signal amplification through thermal cyclic primer extension
To increase the sensitivity of mass cytometry analysis, synthetic oligonucleotides were used to create repeats of metal probe hybridization sites using thermal cyclic extension, amplifying the number of metal ions carried by an antibody (Fig. 1a). Antibodies targeting the protein of interest were first conjugated to short DNA oligonucleotide initiators (TT-a, in total 11-mer, a = 9-mer). Mixtures of conjugated antibodies were then applied to cell suspensions for cell surface or intracellular marker staining (step 1). Next, an extender oligonucleotide containing two repeats of sequence complementary to the initiator, separated by a deoxythymidine spacer (a*-T-a*, 19-mer) was introduced to the stained cells. At 22 °C, the extender and initiator hybridize, allowing Bst polymerase-mediated initiator strand extension (forming TT-a-A-a-A, step 2, Bst polymerase adds a single A base to the 3′ end after each round of primer extension). Following this, the reaction temperature was raised to 58 °C, to denature the initiator–extender hybrid, and expose the extended initiator strand (TT-a-A-a-A, step 3). These thermal cycles (1 min per cycle) were then repeated to successively elongate the initiator (step 4), creating hundreds of a-A repeats on each antibody conjugation site (step 5). Detectors containing the sequence a*-T-a* were then conjugated to DTPA polymers containing chelated Ln3+ metal ions through a maleimide-thiol reaction. Hundreds of metal-conjugated detectors were hybridized to each extended initiator (step 6), thereby substantially amplifying the metal signal on a per antibody basis.
a, Schematic of the ACE approach for signal amplification in mass cytometry analysis. b, ACE amplifies the signal in suspension mass cytometry, allowing detection and quantification of low-abundance markers that do not reach detection limits of a mass cytometer using conventional approaches. c, Coupled with IMC, ACE enables high-sensitivity multiparametric spatial profiling of healthy and diseased tissue samples.
Initial experiments applying ACE for mass cytometry resulted in low signals and high acquisition backgrounds (Supplementary Fig. 1a, top right panel), suggesting that metal signals were leaking from measured cells. In experiments using fluorescently labeled detectors that could be analyzed by conventional flow cytometry, loss of signal from the detector was reproduced by heating cells for 1 min at 55 °C after ACE, resulting in a greater than 90% fluorescent signal decrease compared with the unheated samples (Supplementary Fig. 1b). In mass cytometry analysis, heat-mediated vaporization of single-cell droplets during the acquisition step denatures DNA double helices and detaches hybridized metal-conjugated detectors, compromising the power of amplification by ACE. To address the above issue, we introduced a photocrosslinking method that incorporates a CNVK24,25 modification in the metal-conjugated detector oligonucleotide. Following the detector hybridization step, a brief (1 s) exposure to ultraviolet (UV) light activates the CNVK photocrosslinker, forming a covalent bond between the detector and a deoxythymidine nucleotide on the complementary hybridized DNA strand (Fig. 1, step 7, and Supplementary Fig 1a, middle panel). This stabilized the ACE amplificaiton complex against heat-induced double-helix denaturing, allowing it to remain intact during 55 °C incubation as assessed by flow cytometry (Supplementary Fig. 1b), and during mass cytometry vaporization (Fig. 1, step 8, and Supplementary Fig. 1a, bottom right panel). The ACE approach can be used on protein epitopes of interest in suspension mass cytometry to facilitate low-abundance marker quantification in single-cell samples (Fig. 1b), or can be applied to imaging mass cytometry to enable highly sensitive spatial analysis of tissue specimens (Fig. 1c). Compared with conventional mass cytometry workflows, the ACE protocol (Supplementary Fig. 2a) requires a total additional cost of ~US$24 for a 30-target amplification experiment (Supplementary Fig. 2b).
ACE enables high-sensitivity mass cytometry analysis
To explore the specificity and amplification power of ACE, we applied ACE to human embryonic kidney HEK293T cells transiently transfected with a plasmid encoding the green fluorescent protein (GFP). Transient transfection yields a high GFP expression gradient, which allows assessment of ACE to quantify target proteins with different abundance (Fig. 2a). A side-by-side comparison between ACE amplification, conventional fluorescently labeled antibodies and immuno-SABER amplification on flow cytometry revealed that the signal-to-noise ratio in ACE was 3.6-fold higher than that obtained by secondary antibody amplification, and 27-fold higher in comparison with immuno-SABER amplifcation21 (Supplementary Fig. 3).
a, HEK293T cells transiently transfected with a GFP-encoding plasmid were used to validate the ACE amplification method. b, Signal amplification by ACE (y axis) through 1–500 thermal cycles was compared with counterstaining using a secondary antibody conjugated with the conventional protocol (x axis) to validate the specificity and quantify the amplification efficiency of ACE. Pearson correlation coefficients between ACE and the regular secondary antibody signals were calculated for each condition as a measure of ACE specificity. c, Data were divided into ten equal-width bins according to their GFP expression levels shown by the secondary antibody. Bins 1–3 reflect the untransfected cells as internal controls. Bin 10 shows the cells with the highest GFP expression levels. d, Bin medians across 1–500 thermal cycles. e, Ratio of each bin median over the median in bin 10 across 1–500 thermal cycles. f, GFP ion counts generated with ACE after one or two rounds of branching were compared with that from a linear amplification without branching in a ERK2–GFP transient expression experiment using ultralow amount of the GFP antibody (10 ng ml−1). A conventionally conjugated anti-ERK2 antibody was used as counterstaining. g, To examine the orthogonality of ACE, GFP-expressing HEK293T cells were stained individually with anti-GFP ACE antibodies conjugated to 33 initiator sequences before they were barcoded, pooled and processed through the ACE protocol in the same tube, and then analyzed on a mass cytometer. h, Data were then debarcoded to allow pairwise analysis of potential ACE crosstalk. Ion counts generated by a detector in all conditions (columns) were normalized to 0–1 before the ratio between any unmatched initiator–detector pair (for example, Ab-initiator 1–Detector 2*-142Nd) and the true signal (for example, Ab-initiator 1–Detector 1*-141Pr) was calculated. On average, ACE has 1.02% of crosstalk signal. Four pairs of probes were detected with crosstalk degrees over 10% (Initiator 2–Detector 3*, Initiator 4–Detector 5*, Initiator 7–Detector 8*, Initiator 20–Detector 21*). 1°, primary; 2°, secondary; Ab, antibody.
Next, GFP-expressing cells were stained with oligonucleotide-conjugated rat anti-GFP antibodies for ACE amplification through 1–500 thermal cycles, followed by 172Yb-labeled detector hybridization. Conventionally labeled 159Tb anti-rat secondary antibodies27 were used to detect the anti-GFP antibody in the same cells, which were later analyzed by mass cytometry (Fig. 2b). In the absence of cyclic amplification (that is, after the first thermal cycle), the ACE signal of the primary anti-GFP antibody is correlated strongly with the secondary anti-rat antibody, which was metal-conjugated using the conventional protocol (Pearson correlation coefficient = 0.927; Fig. 2b), validating the specificity of the ACE for intracellular epitope staining. Repeated thermal cycles resulted in a continual increase in the ACE 172Yb signal from GFP-positive cells relative to the 159Tb-labeled secondary antibody, while the signal from GFP negative cells remained at the same levels. Notably, this led to increased Pearson correlations (to a maximum of 0.975) between ACE and the regular secondary antibody (Fig. 2b). We then discretized the data according to the secondary antibody signal (x axis) into ten equal-width bins (Fig. 2c and Supplementary Fig. 4a), and calculated the bin medians over thermal cycles. This revealed that ACE amplification was most efficient in the first 100 cycles (about 2 h), after which the amplification efficiency declined over time (Fig. 2d). A 13-fold amplification strength and a sixfold enhancement of signal-to-noise ratio were observed in samples with 500-cycle amplification, compared with the unamplified control (Supplementary Fig. 4b). Untransfected cells (GFP negative, bins 1–3) were not amplified through the timeseries, confirming the specificity of our approach (Supplementary Fig. 4b). The consistency of signal ratios in relation to the highest signal (median levels in bin 10) was maintained throughout the 500-cycle amplification process, indicating that ACE amplification does not introduce bias into the linearity of mass cytometry signals (Fig. 2e). We further validated that the thermal cycles did not affect signals of prestained conventional mass cytometry antibodies (Supplementary Fig. 4c,d). After cyclic extension, DNA concatemers were shown to be stable at 4 °C, which suggests high reproducibility despite varied sample storage time (Supplementary Fig. 4e). We also demonstrated that ACE is compatible with low-parametric flow cytometric analysis by applying fluorescently labeled detectors instead of metal detectors (Supplementary Fig. 4f).
The sensitivity of ACE can be further improved by applying branching amplification. After the primary cyclic extension, branching primers (a*-T-a*–b) were applied and crosslinked to the concatemers. Extending the branching primers through additional thermal cycles generated increased numbers of detector binding sites (Supplementary Fig. 5a). We observed that branching amplification with 50 thermal cycles generated a further ninefold signal enhancement, compared with linear amplification (Fig. 2f and Supplementary Fig. 5a). In addition, a secondary branching approach could also be applied, resulting in an additional fivefold signal increase, and a total 500-fold increase from an initial unamplified signal (Fig. 2f and Supplementary Fig. 5a). This allowed us to quantify the GFP protein expression levels in single cells as small as Escherichia coli (Supplementary Fig. 5b).
Taken together, these observations indicate that the ACE amplification method should enable mass cytometry to measure a broad range of low-abundance proteomic epitopes that have been challenging to assess at the single-cell resolution9,10,11.
Multiparametric amplification using orthogonal ACE barcodes
To use ACE for simultaneous multiparametric signal amplification, we adapted our previously designed nine-mer orthogonal oligonucleotide sequence library20 to identify ACE initiator strands that could be extended by a corresponding set of 19-mer extender strands (Supplementary Table 1). We conjugated 37 CNVK modified detectors with metal isotope chelation polymers and validated 30 branching primers (Supplementary Table 1). As an experimental assessment of orthogonality, 33 initiator strands were labeled individually on the anti-GFP antibody (Fig. 2g). GFP-expressing HEK293T cells were stained with each of the 33 GFP antibodies in separate tubes before they were mass-tag barcoded3,28 and pooled for simultaneous ACE extension and detector hybridization in one tube. After the mass cytometry measurement, the degree of crosstalk was assessed by calculating the ratio between an unmatched crosstalk signal (that is, signal resulted from unmatched initiator and detectors) compared with that of a true matched signal (Fig. 2h). An average crosstalk signal of only 1.02% was observed, indicating that most of the initiator sequences could be extended and detected only by their corresponding extenders and detectors. We detected four pairs of probes with crosstalk degrees over 10% (Fig. 2h; Initiator 2–Detector 3′, Initiator 4–Detector 5*, Initiator 7–Detector 8*, Initiator 20–Detector 21*). These high-crosstalk pairs were generated between adjacent mass channels, suggesting that mass resolution issues (that is, signal spillover from the +1 or the −1 channel, an inherent artifact in mass cytometry analysis, independent of the oligonucleotide orthogonality)29 contributed partially to the observed crosstalk. Multiparametric ACE antibody panels were designed by considering the calculated crosstalk matrix (Supplementary Table 2) to minimize the potential influence of channel-to-channel spillovers.
Single-cell assessment of low-abundance transcription factors
A 32-parameter ACE antibody panel (Supplementary Table 3) was created to profile signaling molecules, cell phenotypic markers and transcription regulators during the EMT and a reverse MET process in mouse breast cancer Py2T cells26. Epithelial Py2T cells were treated with 4 ng ml−1 transforming growth factor (TGF)β1 for 14 days to induce a mesenchymal transition, which was then followed by a 14-day timecourse after TGFβ was withdrawn, allowing cells to revert back to an epithelial state (Fig. 3a). Cells were harvested at 11 timepoints during the EMT–MET timeline, mass-tag barcoded and pooled into a single tube. Following fixation and permeabilization, labeling and amplification of the entire panel of ACE-conjugated antibodies was performed on the pooled single-cell sample and the cells were analyzed on a mass cytometer (Fig. 3a). We then performed a dimensional reduction analysis using uniform manifold approximation and projection (UMAP)30 to allow visualization of all cells through the EMT–MET processes in two-dimensional space (Fig. 3a–c). Cell phenotypical modulations and molecular profiles across the timeseries were assessed on the UMAP plots (Fig. 3b,c). The distributions of single cells in all 32 channels, measured over the timeseries, were shown as violin plots (Supplementary Fig. 6). ACE enabled the uncovering of molecular signatures involving low-abundance transcription factors Zeb1 and Snail/Slug during the EMT and MET transitioning process. Zeb1 expression increased sharply starting at day 6 after TGFβ1 treatment, whereas the levels of Snail/Slug showed a slow modest decline during the first 3 days with a secondary peak at 6 days during the EMT process (Fig. 3b,c and Supplementary Fig. 6). Along with transcriptional reprograming, the expression levels of epithelial markers, E-cadherin, CK14, EpCAM and β-catenin declined during the TGFβ1 treatment and increased upon TGFβ1 withdrawal. Mesenchymal markers vimentin and CD44 increased during TGFβ1 treatment, peaked at 14 and 9 days, respectively, and gradually decreased after TGFβ1 was removed (Fig. 3b,c and Supplementary Fig. 6). Interestingly, the proliferative signals from p-ERK1/2 and its downstream substrate p-p90RSK were reduced during the epithelial to mesenchymal transition, and increased again during the mesenchymal to epithelial transition, closely paralleling similar changes in EGFR expression (Fig. 3b,c and Supplementary Fig. 6).
a, Experimental workflow: mouse breast cancer Py2T cells were treated with TGFβ1 (4 ng ml−1) for 14 days before the stimulus was withdrawn. Cells were cultured in the absence of TGFβ1 for an additional 14 days. During the timecourse, Py2T cells were harvested on the days indicated posttreatment, barcoded and pooled into a single vial for simultaneous ACE amplification and subsequent mass cytometry analysis. Experiments were performed in biological replicates to confirm reproducibility. b,c, Dimensional reduction analysis with UMAP was performed on the data. Cells are color coded by treatment time (b) or abundance of measured markers after normalization (c) on UMAP plots. d, Pseudotime analysis with Scorpius was performed and plotted against the actual time posttreatment in a violin plot. e, Signed Scorpius analysis was used to study the molecular modulation trajectories of measured markers during the EMT–MET transitioning. f, Biaxial plots show the abundances of Zeb1 and cyclin B1 levels in each single cell during the MET process across the five indicated timepoints. Dashed lines indicate the gating strategy to distinguish the Zeb1 high cyclin B1 low populations and the Zeb1 low cyclin B1 high populations. g, Boxplots showing expression levels of a mesenchymal marker vimentin and two epithelial markers, E-cadherin and CK14, in the Zeb1 high cyclin B1 low populations and the Zeb1 low cyclin B1 high populations across the five MET timepoints. Boxplots present the first quartile (Q1), median and third quartile (Q3). The interquartile range (IQR) defines distance between Q1 and Q3. The upper whisker extends from the hinge to the largest value no further than 1.5 × IQR from the hinge. The lower whisker extends from the hinge to the smallest value no further than 1.5 × IQR of the hinge. Data beyond the end of the whiskers are plotted individually.
Next, we applied an unsupervised trajectory inference method, Scorpius31, to reconstruct the EMT–MET in pseudotime. Comparing EMT–MET pseudotime with the actual time (Fig. 3d), we found that the molecularly defined mesenchymal phenotype started to appear at day 6, followed by the complete diminishment of the molecularly defined epithelial phenotype 9 days after TGFβ1 stimulation. During MET, cells with epithelial molecular properties expanded slowly through the 14-day timeseries while a large population retained its mesenchymal cell state, suggesting that some component of the emerging epithelial population arises in MET cells undergoing clonal expansion (Fig. 3d). The application of ACE allowed coupling the quantification of low-abundance transcription factors to other phenotypical markers. By analyzing measured markers through signed Scorpius pseudotime analysis (Methods), we confirmed that the gain of Zeb1 expression occurs during the late EMT phase in cells with downregulated CK14. Zeb1 expression was linked closely with vimentin upregulation and the quick decline of E-cadherin. During the reverse MET process, the decline of Zeb1 expression correlated with a sharp decrease in vimentin expression, which was preceded by a rise in the levels of E-cadherin (Fig. 3e). Our method enabled the discovery of an expanding population of cells over the MET timecourse with low Zeb1 and high cyclin B1 expression levels (Fig. 3f). Only these cells showed decreased vimentin, increased E-cadherin and CK14 expression levels (Fig. 3g), suggesting that suppression of Zeb1 and the gain of cyclin B1 are hallmarks for cells undergoing a MET.
Overall, these analyses demonstrate the capability of ACE to simultaneously amplify a large number of metal channels used in mass cytometry, allowing the accurate profiling of low-abundance proteomic markers for cell state transitions like EMT and MET.
Profiling signaling network dynamics in single T lymphocytes
Systematic profiling of TCR signaling networks at single-cell resolution has been technically challenging due to the limited abundance of phosphoproteins in T lymphocytes and their small size (a T cell is 23 times smaller than a HeLa cell, on average). Most of the phosphorylation sites on TCR signaling proteins, particularly at their basal states, do not reach the detection limit of a mass cytometer or result in signals only slightly above the limit32,33,34. We investigated whether ACE could be used to address these limitations and provide a technology capable of single-cell TCR network analysis.
We created and validated a 30-parameter ACE antibody panel that included p-CD3ζ (CD247), p-CD28, p-ZAP70/SYK, p-LAT, p-SLP76, p-PLCγ1 and p-BTK/ITK in the canonical TCR pathways, p-MEK1/2, p-ERK1/2, p-p90RSK and p-S6 in the MAPK-ERK-related pathways, and many phosphorylation sites involved in stress, inflammation and cell cycle regulation (Supplementary Table 4). We first examined the ability of this multiparametric ACE antibody panel to report on TCR signaling events using human Jurkat T cells harvested over a 1-h TCR stimulation timecourse (Supplementary Fig. 7). Compared with DNA-antibody without amplification, ACE enhanced signals of measured phosphorylation sites with an average amplification power of 17 times (Fig. 4a and Supplementary Fig. 7a,b) and an increase of dynamic range by tenfold (Supplementary Fig. 7c–e). Compared with conventionally labeled mass cytometry antibodies, ACE enabled signal amplification of seven times (Supplementary Fig. 8a). For the activating phosphorylation site on AKT, phosphothreonine 308, which did not show sufficient signal (ion counts) after linear ACE amplification, we performed one round of branching ACE to further increase the detection sensitivity by 15-fold (Fig. 4a).
a, Thirty key markers related to the TCR signaling network in the human Jurkat T cells were measured with or without ACE amplification and plotted as green or blue boxes, respectively. Mean ion counts for unamplified and linear amplified staining were indicated by the green and blue points with lines between them color coded according to amplification fold change. Boxplots present the first quartile (Q1), median and third quartile (Q3) in unamplified or linear amplified data. The IQR defines the distance between Q1 and Q3. The upper whisker extends from the hinge to the largest value no further than 1.5 × IQR from the hinge. The lower whisker extends from the hinge to the smallest value no further than 1.5 × IQR of the hinge. ACE enabled, on average, 17-fold and a maximum of 41-fold signal amplification. The signal from p-AKT (p-T308) was analyzed with an additional ACE branching amplification (shown as a red box) with 15-fold higher p-AKT (p-T308) counts after the branching amplification. b, Jurkat cells treated with anti-CD3 and anti-CD28 antibodies, and subsequently crosslinked with anti-mouse IgG antibody to activate TCR signaling. Cells were harvested at 0, 0.5, 2, 5, 10, 15, 30 and 60 min after TCR activation. Linear amplification allowed the quantification of key signaling nodes in the TCR network during the 1-h TCR stimulation timecourse. Negative control p-SMAD2 did not show differential phosphorylation levels as measured with ACE. c, Signal responses of p-AKT (p-T308) to TCR activation were analyzed without amplification (bottom), with linear amplification (middle) and with branching amplification (top). Fold changes in the p-AKT signal relative to the first timepoint (unstimulated) were calculated and showed that only with branching ACE could the signaling trajectory be captured. d, Circos plot shows 73 pairs of strong signaling relationships in the TCR signaling networks as detected with the BP-R2 method on the TCR stimulation timecourse data measured by ACE (inner layer). The middle layers show the coefficients of variation of measured phosphorylation sites at all timepoints following TCR stimulation and the signal amplitude for each phosphorylation site. The outer layer demonstrates the signaling trajectory for each marker, characterized by normalized mean ion counts across all timepoints during the TCR stimulation timecourse. Cells used in these analyses were in G1 phase to avoid cell cycle confounding effects. Experiments were performed in biological replicates to confirm reproducibility.
Over the 1-h TCR stimulation timecourse, ACE was able to reveal differential signaling responses on TCR signaling mediators (Fig. 4b and Supplementary Fig. 7b). Our data showed that phosphorylation of CD3ζ and ZAP70/SYK peaked at 0.5 min upon TCR stimulation, consistent with activation of these proteins immediately following TCR stimulation. Notably, indicators of MAPK/ERK pathway activation—p-MEK1/2, p-ERK1/2 and p-p90RSK—showed sustained signaling activities that started to decline only 30 min after TCR stimulation (Fig. 4b). As a negative control, we observed that p-SMAD2 did not respond to TCR stimulation35, further validating the reliability of ACE for measuring specific TCR-induced changes in signaling (Fig. 4b). By analyzing the signaling trajectory of p-AKT (p-T308) over the 1-h TCR stimulation timecourse, we found that branching ACE could uncover a ~1.5-fold signal amplitude on that phosphorylation site. The nonamplified p-AKT (p-T308) antibody lacked the sensitivity to capture the AKT signaling trajectory, which was instead dominated by technical noise (Fig. 4c). To further verify the dynamics of signaling responses captured by ACE, we compared ACE-amplified mass cytometry results with data acquired from flow cytometry using fluorescently labeled conventional antibodies. Phosphorylation levels of p-ZAP70/SYK, p-SLP76 and p-ERK1/2 in Jurkat cells were measured using both approaches and showed similar amplitudes upon stimulation (Supplementary Fig. 8b).
To systematically quantify the strength of signaling relationships in the TCR signaling networks, BP-R2 analysis6 was performed on all pairs of measured phosphorylation sites. This identified 73 strong relationships (BP-R2 above 0.775), which were used to reconstruct a TCR signaling network (Fig. 4d). As expected, the data recapitulated p-ERK1/2 as an essential signaling node in the TCR network, as it connects the upstream TCR responses transduced by p-ZAP70/SYK, p-SLP76 and p-MEK1/2 to downstream effectors, including p-p90RSK and p-S6 (refs. 36,37) (Fig. 4d). Notably, our data also showed that, following TCR engagement, most of the TCR-stimulated phosphorylation sites had a lower coefficient of variation among all of the individual cells compared with the unstimulated control or cells that had returned to steady state 1 h after TCR stimulation (Fig. 4d). This suggests a high degree of basal signaling heterogeneity, which was reduced upon TCR stimulation.
As noted from previous studies32,33 and reproduced in our results, signaling levels and heterogeneity in primary T lymphocytes, especially in their basal states, were more problematic to quantify with conventional mass cytometry compared to Jurkat T cells (Supplementary Fig. 8c). We used branching ACE to amplify signals of TCR-related phosphorylation sites in primary human CD4+ T cells harvested over a 1-h TCR stimulation timecourse and analyzed these cells with mass cytometry. Compared with the conventional approach, ACE enhanced the signals for measured sites by ten times and thus allowed characterization of signaling states and kinetics in primary human T cells (Supplementary Fig. 8c–e).
In summary, our results illustrate that ACE enabled a comprehensive analysis of TCR signaling networks at single-cell resolution, which has been previously considered challenging.
POF samples modulate immunological signaling network responses
Extensive tissue injury caused by trauma or major surgical procedures has long been linked to the late development of an immunosuppressive state, characterized by prolonged inflammation, refractory T cell responses and some degree of T cell death38,39,40. This phenomenon of injury-induced T cell paralysis and death appears to be induced by some combination of wound-related cytokines/chemokines and/or mononuclear cells, although the process is incompletely understood41,42. We applied ACE to further characterize the inhibitory regulation of T cell signaling in response to cues originating in the environment surrounding sites of tissue injury by coculturing T cells with human patient postoperative drainage fluid (POF). The immunosuppressive properties of the POF samples were first assessed using dye-based T cell proliferation assays. Signaling responses were subsequently assessed using the immune-based 30-parameter ACE protein phosphorylation panel (Fig. 5a).
a, Proliferation and TCR signaling effects of POF on human T lymphocytes were assessed using dye dilution and flow cytometry, and through the ACE-based 30-plex TCR signaling mass cytometry analysis, respectively. b, Percentage of proliferative T lymphocytes after treatment with anti-CD3/anti-CD28 beads or with indicated POF samples. Data are presented as mean values ± s.d.; n = 3 individual experiments. ***P < 0.001 using a two-tailed t-test); ***P value = 0.0000832. c, TCR stimulation was performed in the presence of POF or medium control. The cells were fixed 0, 2, 5, 10, 15 and 30 min later, and analyzed by ACE. The timepoint when the maximal signal for each specific phosphorylation site was observed after TCR stimulation is shown. d, The integrated signal corresponding to each phosphorylation site across the stimulation timecourse was calculated as the AUC/HMP. This differs across all POF treatment conditions and the medium-only control as indicated by circle size and color. e, Signaling trajectories for p-CD3ζ, p-ZAP70, p-SLP76, p-BTK/ITK, p-ERK1/2 and p-S6 for each POF sample and the medium-only control are shown. Dashed lines indicate HMP signals; shading defines the AUC/HMP.
We found POF1 reduced T cell proliferation, compared with the medium-only control, suggesting a highly immunosuppressive environment caused by this POF sample. POF2 inhibited T cell proliferation to a lesser degree than POF1, consistent with moderate immunosuppression, whereas POF3 did not demonstrate any T cell inhibitory effects (Fig. 5b and Supplementary Fig. 9).
We next analyzed the signaling dynamics of measured phosphorylation sites in the TCR signaling network profiling using ACE, and characterized the peak signaling times and total signaling integrals (that is, areas between the signaling trajectory (AUC) and half-maximal point (HMP); Fig. 5c–e; Methods). In most of the proximal TCR signaling and MAPK/ERK nodes, including p-ZAP70/SYK; p-SLP76; p-PLC-γ1; p-BTK/ITK; p-STAT-1, -3 and -5; p-MEK1/2; p-ERK1/2; p-p90RSK and p-S6, we observed a decrease in total signaling responses and a shift towards earlier peak times in POF1 and POF2 cotreated samples, compared with the medium-only control and POF3 cotreatment (Fig. 5c–e). Similarly, POF1 and 2 cotreatments resulted in suppression of cyclin E, cyclin B, p-CDK1 and p-HH3 compared with POF3 and the medium control. Taken together, these data indicate that POF1 and POF2 cotreatment caused a reduced and more transient TCR signaling response to anti-CD3/anti-CD28, leading to a net reduction in proliferative signals compared with the control. These findings are consistent with a requirement for sustained MAPK/ERK activation for T cell proliferation43,44, in good agreement with our observations from the T cell proliferation assay (Fig. 5b and Supplementary Fig. 9). Finally, these results demonstrate that ACE-based TCR signaling network profiling can be used to explore molecular mechanisms underlying immunosuppression after tissue injury at single-cell resolution, with the potential to identify targets for future therapeutic intervention.
Signal amplification for IMC multiparametric spatial analyses
Substantial autofluorescence of renal tissue45 restricts the use of fluorescence-based multiparametric imaging approaches46,47,48 in the spatial profiling of proteins in histologic sections of the human kidney. IMC analysis is void of sample autofluorescence effects7. However, due to problems with instrument sensitivity and the lack of proper signal amplification methods, it has been reported that many antibodies showed poor signals on IMC despite their good performances in immunohistochemistry11. As another application of ACE, we coupled our amplification approach with IMC to demonstrate a highly sensitive multiparametric spatial analysis for human kidney protein markers.
We established a 20-antibody panel, which included markers commonly used in immunohistochemistry, to assess human renal disorders (Supplementary Table 5). Validated antibodies were applied to a cryosection slide of the renal cortex from a patient with polycystic kidney disease, followed by a linear ACE amplification for all markers. We applied branching amplification to ten low-abundance kidney markers, further amplifying their signals for enhanced detection (Supplementary Fig. 10a,b and Supplementary Table 5). After the detector hybridization, signals were acquired from the samples using an IMC instrument (Fig. 6a), reconstructed as multiparametric images (Supplementary Fig. 10c), and are displayed as overlaid images (Fig. 6b). Fibronectin and α-smooth muscle actin (SMA) were observed in vesicular structures with three layers of mutually exclusive expression profiles: an inner layer of fibronectin+ endothelial cells and a middle layer of vesicular smooth muscle cells (α-SMA+) surrounded by an outer layer of fibroblasts (fibronectin+) (Fig. 6b and Supplementary Fig. 10c). Epithelial cells in the kidney tissue showed differential staining patterns for various markers including EpCAM, ATP1A1, uromodulin, E-cadherin, aquaporin1 and aquaporin2. The presence of the tubular segment-specific makers, aquaporin1, uromodulin or aquaporin2 identified cells in the proximal tubules, distal convoluted tubules and collecting ducts, respectively. The abundance of ATP1A1 varied across the tubular system, with stronger expression levels seen in the distal convoluted tubules and collecting ducts, compared with the levels of ATP1A1 in proximal tubules (Fig. 6b and Supplementary Fig. 10c). Vimentin, nephrin, fibronectin and CD31 were positive in clusters of cells through the section, revealing the structure of glomeruli. Nestin expression was detected with high heterogeneity in these glomeruli (Fig. 6b and Supplementary Fig. 10c). Strongly upregulated nestin levels indicate the gain of stemness in repopulating mesangial cells in kidney disorders49,50,51. The variance of glomeruli nestin levels in our sample potentially suggests different pathological stages of injury and repair within the same tissue.
a, Renal cortex tissue from a patient with polycystic kidney disease was used to demonstrate the application of ACE for IMC-based tissue imaging. Thermal-cycling reactions were performed on glass slides. b, Simultaneously measured IMC channels were overlaid to demonstrate the high specificity of ACE in spatial tissue protein profiling. c, After single-cell segmentation, dimensional reduction analysis with UMAP was performed to project cells on a two-dimensional space. Cells are color coded by normalized abundances of measured markers on the plots. d, Using the Phenograph algorithm, 18 clusters of cells were identified and color coded on the UMAP plot. These cell classifications suggested phenotypical heterogeneity in six main renal tissue compartments (encircled with dashed lines). e, The mean expression level of each marker was computed for each cell cluster and is shown as a heatmap. f, The identity of every single cell as classified by Phenograph was illustrated on the segmentation mask to generate a pseudoimage showing the kidney tissue organization by identified cell type. Experiments were performed in replicates to confirm reproducibility. Scale bar, 200 μm.
To further classify and quantify the cells profiled in our ACE–IMC analysis, we performed single-cell segmentation analysis using Mesmer52, and embedded the segmented cells into a two-dimensional UMAP plot that was colored by the expression level of each measured maker (Fig. 6c) or by the cell clusters identified using the Phenograph algorithm5 (Fig. 6d). We then computed the average abundance of each marker over all identified clusters (Fig. 6e). Cluster identities were also mapped to the single-cell mask to generate a pseudoimage colored by detected cell types (Fig. 6f). These analyses identified the glomerular compartment within the analyzed kidney section consisting of cells in cluster 17, with a subpopulation showing high nestin expression levels—a potential biomarker of mesangial expansion in this patient with polycystic kidney disease (Fig. 6c,d). The blood vessel compartment was identified by the presence of endothelial cells and smooth muscle cells, indicated by clusters 1 and 13, respectively (Fig. 6c,d). In addition, our analyses delineated proximal tubules, distal convoluted tubules and collecting ducts in the renal tubular system that were well distinguished on the UMAP plot (Fig. 6d). Marker expression profiles varied within these tissue compartment (Fig. 6c,e), indicating tissue heterogeneity that could potentially lead to more defined cell type classification. Finally, a resident fibroblast compartment with high COL1A1 expression levels was also detected in our analysis (Fig. 6c,d and Supplementary Fig. 10c).
Overall, our results demonstrate the application of ACE to IMC-based multiparametric tissue imaging with enhanced sensitivity. We recapitulated known tissue compartments in the human kidney and revealed various spatial aspects of the pathological state in diseased human tissue.