Study design and participants
The TransEuro Transplant trial (NCT01898390) was a randomized, open-label study that recruited patients from five sites across the United Kingdom and Sweden (University of Cambridge, Cambridge, UK, Imperial College London, London, UK, the National Hospital for Neurology and Neurosurgery, London, UK, University of Cardiff, Cardiff, UK, and Skåne University Hospital, Lund, Sweden). Eligible participants were recruited from the ongoing observational TransEuro observational study9 and rescreened using the original observational study inclusion criteria with modifications (Supplementary Table 5) to ensure continued eligibility for transplantation. Thirty-six patients were recruited into the transplant trial and randomly allocated to the transplant arm of the study or the control arm. Six withdrew before any intervention, and three did not complete screening. Eleven patients, of the remaining 27, went on to receive hfVM transplants. Sixteen patients served as a control arm. These patients underwent the same PET and clinical examinations as the transplant arm but did not receive immunosuppression and did not undergo sham surgery.
Ethical approval
Ethical permission was received for fetal tissue preparation and use at Cambridge (96/085), Cardiff (13/WA/0210ADD) and Lund (2013/432 and 2016/535). The transplant study was approved by the relevant ethical authorities in the United Kingdom and Sweden (REC reference number 10/H0304/77 in the United Kingdom and the Swedish Ethical Review Authority (Etikprövningsmyndigheten) in Lund (reference numbers 2011/290, 2014/877 and 2019-06529)).
Changes in study design
The original study design was to transplant 20 patients in an open-label fashion, drawn from a larger natural history cohort of 150 participants, and the data obtained would then be used to calculate sample size needed for a larger double-blind placebo-controled trial. All of this was to be done over a 5-year period. However, 5 years into the natural history study, the first patient was grafted, and, at this point, a decision was made to stop the transplant trial when either all 20 patients had been grafted or 3 years had elapsed from the time of the first transplant. This decision was based on the following reasons:
-
(1)
it seemed unethical to prolong the study beyond this time as it was clearly showing that using this tissue source in a trial was not feasible in the United Kingdom and Sweden,
-
(2)
interpretation of the study would become extremely difficult if some patients had already reached their primary endpoint whereas others had still not been grafted, and
-
(3)
advances in human stem cell-derived dopamine cells meant that trials using this new source of more readily available cells were already entering the clinic24.
At the end of 3 years in 2018, a total of only 11 patients had been grafted (8 in Cambridge, UK, and 3 in Lund, Sweden). The reasons for this have been previously presented9. Thus, the time of the final data collection for our predefined primary endpoint for the last patient was in March 2021. Collection of the follow-up clinical and PET imaging data was delayed (and in some cases not possible) because of restrictions resulting from the COVID-19 pandemic that began in March 2020, and this included the 36-month PET imaging in the majority of patients. To try and standardize the timings to better align with the PET imaging analysis, we defined a pretransplant baseline as the visit immediately before the first transplant. We then elected to use the following as the key time points for the primary and secondary outcomes:
-
18 months: first visit at least 510 days after the last transplant surgery or after the baseline visit (control; 540 days = 30 × 18 months with 30 days leeway)
-
36 months: first visit at least 1,020 days after surgery (transplant) or after the baseline visit (control; 1,080 days = 30 × 36 months with 60 days leeway)
Clinical assessments
Once randomized, participants continued trial visits as part of the observational TransEuro trial schedule every 6 months. During each visit, participants underwent a battery of clinical tests during an OFF state (with the OFF state being defined as the patient not having had any dopaminergic medications for 12 h before assessments or 24 h for long-acting dopamine agonists) and ON state (defined as at least 1 h after the patient had taken their regular morning dose medications). These assessments included UPDRS Part III, RUSH Dyskinesia Scale and AIMS. Patients also completed the UPDRS Parts I, II and IV, Addenbrookes’ Cognitive Examination-Revised and a series of other cognitive and PD-related assessments (Supplementary Table 6). Study data were collected and managed using REDCap electronic data capture tools hosted at the University of Cambridge.
Imaging assessments
MRI and PET scanning were performed at Invicro, Hammersmith Hospital, London, UK. Patients had a structural MRI and [11C]PE2I, [11C]DASB and [18F]FDOPA PET scans at baseline and repeat scans just before surgery and at 18 months after their first transplant. The planned 36-month scanning could not be performed in sufficient numbers of patients because of the COVID-19 pandemic that began in 2020.
Image processing and kinetic modeling were conducted using MIAKAT v4.3.13 (Molecular Imaging and Kinetic Analysis Toolbox)25 implemented within MATLAB 2016b (Mathworks), SPM12 v7487 (Statistical Parametric Mapping, Wellcome Trust Centre for Neuroimaging) and FSL v6.0 (FMRIB Image Analysis Group)26.
Structural MPRAGE images were segmented and rigid registered to the MNI template, and for each patient, all visits were entered into serial longitudinal registration to create a midpoint average and associated deformation fields. The midpoint was used to define the putamen and caudate in accordance with previously published anatomy-based guidelines27. Cerebellar gray matter was isolated using DARTEL to estimate flow fields from the MNI template to native space, applying this to CIC Atlas v1.2 and masking with a gray matter segment. Dynamic PET images were motion corrected and co-registered with their corresponding MPRAGE, using the summed PET images as an intermediary and normalized mutual information as a cost function, in one interpolation step. Parcellations were then applied to the dynamic PET data to generate regional time–activity curves. For [18F]FDOPA, Patlak graphical analysis was used to quantify the uptake rate constant (Ki), whereas for [11C]PE2I, Logan graphical analysis was used to quantify BPND. For both, t* was set to 30 min. For [11C]DASB, BPND was estimated with Simplified Reference Tissue Model 2 (SRTM2). Cerebellar gray matter was used as a reference region for all tracers.
Tissue preparation
Transplanted tissue was prepared from hfVM dissected from three fetuses collected after either medical or surgical abortions under full ethical approval. Tissue dissections were standardized across centers by established landmarks and documentation of each cut using photographs. The landmarks used for dissection are shown in Supplementary Fig. 1.
The collected tissue was stored for a maximum of 4 days in Hib(E) at 4 °C. On the day of surgery, three hfVMs were pooled and washed several times in DMEM (cGMP compliant, Life Technologies, A12861 01)/tirilazad mesylate (custom made to GMP grade, Rechon Life Sciences). The hfVMs were enzymatically digested in a mixture of Tryple E CTS (cGMP compliant, Life Technologies A12859-01) and Pulmozyme (Dornase-α, Roche) at 37 °C for 20 min. After incubation, the tissue was washed three to four times in dissociation medium DMEM/tirilazad mesylate/dornase-α to remove any Tryple E residue. The hfVMs were then dissociated very gently to produce a crude cell suspension, which was spun down and resuspended. Aliquots of 5 × 20 µl were prepared after confirmation of viability and transported to theater in a temperature-monitored cool box. Quality criteria to proceed with transplantation of hfVM cells was set at >80% cell viability on the day of implantation. Although insufficient tissue was a common problem given that the cell preparation had to be derived from at least three hfVMs per side grafted, only one cell preparation had a viability below that required for surgery. The crown rump length of the fetus varied between 15 mm (gestational age (weeks ± days) 7 + 6) and 35 mm (gestational age 10 + 2), and the final cell suspension viability was between 83 and 93%.
It is worth noting that in TransEuro, unlike previous hfVM transplant trials, the tissue was dissociated not using trypsin but using Pulmozyme, as the former could not be sourced at a clinical grade. Given the low number of patients transplanted, it could not be experimentally determined whether Pulmozyme was superior or not to trypsin and had impacted the final number of dopamine cells in the grafted tissue. In addition, we used tissue collected from medical terminations of pregnancy, not surgical terminations, as was the case in earlier trials28. This may also have had an impact on the final number of surviving dopaminergic cells within the graft, as might the time spent in hibernation media before the final tissue preparation and transplant surgery.
Surgery
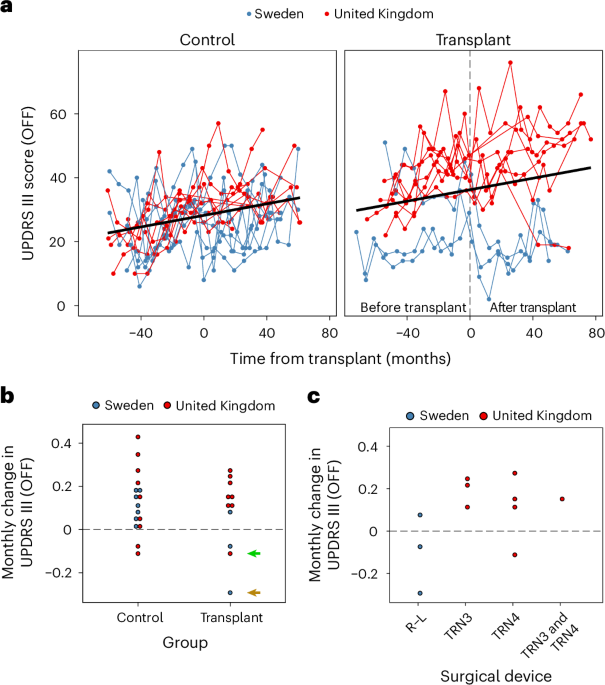
Neurosurgery was performed at one of two sites. All three Swedish patients underwent surgery at Skåne University Hospital, Lund, Sweden. All eight patients recruited at the UK sites had surgery performed at Cambridge University Hospital, Cambridge, UK. Each patient underwent two unilateral transplants within an interval of 1–5 months (3.88 ± 2.49 months) with imaging guidance for trajectory and stereotactic planning. Five trajectories were made per putamen using a transfrontal approach. Eight deposits of 2.5 μl were injected per trajectory for a total of 20 μl per trajectory. MRIs were performed after surgery to show the sites of tissue deposition (Supplementary Fig. 2), although only the needle tracts can be seen, not the transplant itself, as MRI cannot provide validated evidence for the integration of the grafted cells into the brain. Due to regulatory differences between countries, different surgical devices were used to deliver the transplants between the UK site and the Swedish site. The device used in Lund was the original R–L device used in previous open-label trials28, whereas in Cambridge, an in-house-manufactured version of this device was made (TRN3), which was subsequently modified (TRN4) part way through the trial. This modification was undertaken in response to feedback from the neurosurgeon using the device in Cambridge, and the needle in both devices had an internal diameter of 0.82 mm and an external diameter of 1.07 mm.
After surgery, patients were given prophylactic antibiotics and were started on a standard whole-organ immunosuppressant regimen of cyclosporin (titrated to serum levels of between 100 ng ml–1 and 200 ng ml–1), 2 mg per kg (body weight) per day azathioprine and 40 mg of prednisolone weaning to 5 mg over 12 weeks after a one-off dose of 1 g at the time of surgery. Immunotherapy was maintained for 12 months after the last transplant and was then stopped. During this time, patients also took all recommended prophylactic treatments for patients on this immunosuppressive regimen, namely co-trimoxazole three times a week, omeprazole, calcichew daily and alendronic acid once a week.
Postsurgical visits
Patients were followed up 12, 24 and 48 h after surgery for routine postsurgical observations and blood tests. Two days after surgery, a postoperative brain MRI scan was performed to verify graft placement and examined for any perioperative hemorrhage. In addition to their regular study clinical assessment visits, patients also had safety visits at 7, 14, 21, 28 and 42 days and then 2, 3, 4, 5, 6, 9 and 12 months after surgery as well as blood testing to monitor their immunosuppression.
Video rescoring
To maintain intersite and inter-rater reliability, all UPDRS Part III assessments were videotaped. A random selection (n = 25) of these corresponding to the key time points (pretransplant visit and 36-month post-transplant visit or equivalent for controls) were examined by an independent rater blinded to the patient’s transplant status and rescored. These videos did not reveal the patient’s surgical status as all patients were required to wear a hat at all assessments to hide the presence or absence of surgical scars so that their group allocation (transplant or control) could not be identified from the videos. These rescored UPDRS Part III scores were used in the evaluation instead of the original score; however, the overall concordance rate between the two UPDRS scores was high (Supplementary Fig. 3).
Clinical outcomes
The primary outcome measure was defined as change in the UPDRS Part III score in the defined OFF state at 36 months after surgery compared to baseline. As the treatment is a dopamine therapy, and most likely to affect motor outcome, a motor score was felt to be the most appropriate measure, and the 36-month time point was chosen to allow sufficient time for any post-transplant benefits to evolve.
Secondary outcomes included a range of motor, nonmotor, quality of life and cognitive measures as well as changes in dopaminergic medication. These are summarized in the main text.
Statistical analysis
Given the limitations of sample size, site to site variability and procedural and patient heterogeneity, it is debatable whether inferential analyses are relevant and interpretable. Therefore, no inferential statistics were used to assess the efficacy of the transplants on the primary or secondary outcomes.
Statistical analysis of the imaging data included all bilaterally transplanted patients who completed the multi-PET protocol at three time points (n = 8). We performed two-way mixed ANCOVAs with repeated measures to examine whether differences in mean putamenal [18F]FDOPA Ki, [11C]PE2I BPND and [11C]DASB BPND values depended on group (transplant or control) and visit. Visit included pretransplant and 18 months post-transplant time points for the transplant group (n = 8) or baseline and 18-month follow-up time points for the control group, for which data were available for 16 patients for [18F]FDOPA Ki and [11C]PE2I BPND and for 14 patients for [11C]DASB BPND. We also conducted a series of two-way repeated measures ANCOVAs to examine whether differences in mean striatal [18F]FDOPA Ki, [11C]PE2I BPND and [11C]DASB BPND values depended on visit (baseline, pretransplant and post-transplant) and striatal region (putamen and caudate). The caudate was included as an internal control region given that it is also known to exhibit substantial dopaminergic neurodegeneration over time in PD. For both analyses, mean-centered age and disease duration at the first included time point were entered as continuous covariates where possible as they have been shown to be related to dopaminergic and serotonergic loss. Post hoc Tukey-adjusted pairwise comparisons of the estimated marginal means were conducted to evaluate pairwise differences where appropriate.
Spearman’s rank-order correlations were conducted to evaluate the relationship between the changes in PET parameters (posttransplant–pretransplant) and primary and secondary outcome scale change scores. For this purpose, observational data collected closest in time to the PET acquisitions were included for analysis.
Statistical analyses and visualizations were computed in R version 4.2.2 using the following packages: afex 1.3.0, car 3.1.2, emmeans 1.8.6, moments 0.14.1, geoR 1.9.2, rcompanion 2.4.30, rstatix 0.7.2, Hmisc 5.1.0, FSA 0.9.5, ggplot2 3.4.1 and ggpubr 0.6.0.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.