CROPseq-iT7 backbone
CROPseq-Guide-Puro8, which was a gift from Christoph Bock (Addgene, 86708), was PCR amplified with primers CROPseq_iT7_fwd/rev using Q5 polymerase. PCR products were DpnI digested, 5′ phosphorylated and circularized by T4 ligation. Transformants were sequence verified using Tn5-mediated whole-plasmid tagmentation and MiSeq sequencing. The NIS-Seq-compatible CROPseq-iT7 backbone was deposited to Addgene (no. 211699).
Library cloning
Library cloning was performed as previously described by Joung et al.34. In brief, the Human Brunello CRISPR knockout pooled library, a gift from David Root and John Doench (Addgene, 73178)20, was PCR amplified with gRNA_library_fwd/rev primers. The target vector CROPseq-iT7 was digested with the restriction enzyme Esp3I. Subsequently, the purified PCR product was cloned into the digested vector using Gibson assembly. The assembled reactions were pooled and purified via a Zymo DNA Clean & Concetrator-25 column. The purified library was electroporated in eight replicates into Endura electrocompetent cells (Lucigen), using 50–100 ng µl−1 DNA and 25 µl of cells in 0.1-cm Bio-Rad cuvettes at 1,800 V, 10 µF and 600 Ω. Cells were directly recovered in 975 µl of pre-warmed recovery medium and incubated for 1 h at 37 °C and 300-r.p.m. shaking. Then, each culture was transferred to 1 L of LB medium containing 100 µg ml−1 ampicillin and grown overnight at 37 °C and 230-r.p.m. shaking. DNA was purified using a PureLink HiPure Plasmid Maxiprep Kit. Library coverage was determined by Illumina NGS using primers MiSeq_gRNA_fwd_S0-S8, MiSeq_gRNA_rev for target amplification and Barcoding_fwd/rev_1-96 for a secondary barcoding PCR using NEBNext PCR polymerase. The NIS-Seq-compatible CROPseq-iT7 Brunello library was deposited to Addgene (no. 223064).
Tissue culture
HeLa cells and immortalized murine macrophages (iMacs) were cultivated in DMEM GlutaMAX media supplemented with 10% FCS and 10 µg ml−1 ciprofloxacin in a 37 °C incubator with 5% CO2. THP1 cells, murine melanoma (B16-F1), human melanoma (MaMel65) and colon carcinoma (MC-38) cells were cultivated in RPMI GlutaMAX media supplemented with 10% FCS and 10 µg ml−1 ciprofloxacin in a 37 °C incubator with 5% CO2. Primary human monocytes were cultivated for differentiation in RPMI GlutaMAX media supplemented with 10% FCS, 10 µg ml−1 ciprofloxacin (Sigma-Aldrich) and 50 U ml−1 M-CSF (ImmunoTools). THP1 cells expressing ASC–GFP from an NF-κB-dependent promoter were purchased from InvivoGen (thp-ascgfp).
Library transduction and quality control
In total, 1.5 × 107 HEK293T cells were transfected in a 15-cm tissue culture dish using Lipofectamine 2000 (Invitrogen) with 14.1 µg of Brunello_iT7 plasmid library, 7.0 µg of lentiviral packaging plasmid pMD2.G (Addgene, 12259) and 10.6 µg of lentiviral packaging plasmid psPAX2 (Addgene, 12260). After 4–6 h of incubation, the medium was changed. Forty-eight hours later, virus-containing supernatant was filtered through a 0.45-µm filter (Merck Millipore), aliquoted and stored at −80 °C. For each of four screening replicates, 8 × 106 HeLa–Cas9–p65–mNeonGreen cells10 were transduced with 1 ml of lentivirus library and 10 µg ml−1 polybrene (Merck Millipore), aiming for a multiplicity of infection (MOI) of 0.1–0.5. After 1 d, cells were selected and induced with 3 µg ml−1 puromycin and 1 µg ml−1 doxycycline (Cayman Chemicals). Cells were split 1:3 when reaching confluency. For each of two macrophage screening replicates, 1.6 × 107 THP1–ASC–GFP–Cas9 CASP1/8DKO cells were transduced with 2 ml of lentivirus library and 10 µg ml−1 polybrene. The next day, cells were selected with 3 µg ml−1 puromycin. After 4–7 d of selection and induction, 1 million cells were lysed in 100 µl of direct lysis buffer at 65 °C for 10 min and 95 °C for 15 min19. Genomically integrated guide sequences were amplified using NEBNext PCR polymerase (NEB) for 18 cycles and primers MiSeq_gRNA_fwd_S0-S8 and MiSeq_gRNA_rev (Supplementary Table 1) in two replicates per library. After secondary barcoding PCR, purification and NanoDrop-based quantification, libraries were sequenced on an Illumina NextSeq 2000 using a P2 100-cycle cassette. Library members were counted using the online application https://www.jsb-lab.bio/LibCounter.htm.
NIS-Seq
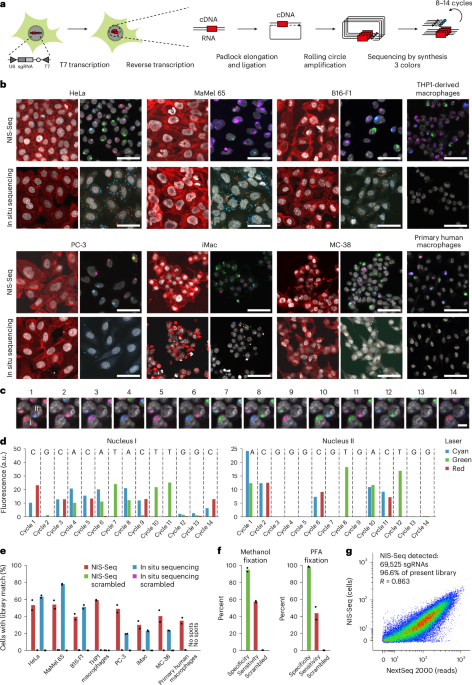
For genome-scale NIS-Seq perturbation screens, 24-well glass-bottom plates (Greiner Bio-One) were coated with 0.1% poly-l-lysine (w/v in water; Sigma-Aldrich) for 30 min at room temperature and washed three times with PBS. For HeLa screens, cells were seeded at 5 d or 18 d of doxycycline induction. Per replicate, 4 × 105 HeLa–Cas9–p65–mNeonGreen Brunello-iT7 library cells were seeded per well and incubated overnight. The next day, cells were stimulated for 45 min with 30 ng ml−1 human recombinant IL-1β (rcyec-hil1b; InvivoGen) or 30 ng ml−1 human recombinant TNF (rcyc-htnfa; InvivoGen), respectively. Live-cell nuclei were stained with 2 µM Hoechst 33342, and membranes were stained with 200 ng ml−1 CellMask Plasma Membrane Stain Deep Red (Thermo Fisher Scientific). For THP1 screens, THP1–ASC–GFP–Cas9 CASP1/8KO Brunello-iT7 cells were pre-differentiated overnight with 100 ng ml−1 phorbol myristate acetate (PMA) (InvivoGen). The next day, cells were washed and detached, and 8 × 105 cells were seeded per well in PLL-coated glass-bottom plates. The next day, cells were primed with 200 ng ml−1 lipopolysaccharide (LPS) (Sigma-Aldrich) for 3 h, pre-incubated with 50 µM Z-VAD (MedChemExpress) for 30 min and stimulated with 7.5 µg ml−1 nigericin (Cayman Chemicals) for 1 h. Cell membranes were stained with 200 ng ml−1CellMask Plasma Membrane Stain Deep Red (Thermo Fisher Scientific). All live-cell phenotype images were acquired in DMEM FluoroBrite with 10 mM HEPES and 2 µM Hoechst 33342 using a ×20 objective for HeLa cells and a ×10 objective with z-stacks for THP1 cells. After phenotyping, cells were fixed and permeabilized with a 3:1 mixture of methanol and acetic acid for 20 min. The fixation was washed out with 1× PBS to avoid dehydration of the cells. Cells were washed with nuclease-free water before adding 200 µl of T7 IVT mix per well (T7 MEGAscript 1× reaction mix; Thermo Fisher Scientific) for 3 h at 37 °C. After IVT, cells were fixed with 4% paraformaldehyde (PFA) in PBS for 20 min and washed with PBS-T (PBS + 0.1% Tween 20) two times. Reverse transcription and post-fixation steps were performed as described by Feldman et al. 10 using oRT_CROPseq_iT7 as reverse transcription primer. After post-fixation, cells were washed three times with PBS-T and incubated with a gap-fill Phusion mix (1× Ampligase buffer, 0.4 U µl−1 RNase H, 100 nM padlock probe oPD_CROPseq_iT7, 0.0125 U µl−1 NEB Phusion polymerase, 0.5 U µl−1 Ampligase, 0.05 mM dNTPs, 0.05 M KCl and 20% formamide) for 30 min at 37 °C and 45 min at 45 °C. Cells were then washed twice with PBS-T and incubated with an RCA mix overnight at 30 °C10. Cells were washed twice with PBS-T before hybridization of 1 µM of the in situ sequencing primer oSBS_CROPseq_iT7 in 2× SSC for 5 min at 37 °C. Cells were washed with MiSeq buffer PR2 (Illumina), and perturbation barcodes were analyzed by sequencing-by-synthesis using reagents from used Illumina NextSeq 2000 P2 cassettes. For each of 14 cycles, cells were incubated with the nucleotide incorporation mix for 3 min at 60 °C, followed by three rounds of five washes with PR2, each with 5-min incubation at 60 °C. The incorporated nucleotides were imaged after the addition of 200 ng ml−1 Hoechst 33342 in PR2. After each imaging cycle, fluorescent nucleotides were cleaved and de-blocked by incubation with NextSeq cleavage mix for 3 min at 60 °C, three washes with PR2, incubation for 2 min at 60 °C and three additional washes before the next cycle of incorporation. Robotic heating steps were performed at 50 °C instead of 60 °C. For a detailed step-by-step protocol, see Supplementary Protocol 1.
Imaging
All images were acquired using a Nikon Ti2 body equipped with a Yokogawa CSU-W1 spinning disc unit connected to Lumencor CELESTA multimode lasers with wavelengths of 405 nm (nuclear staining), 477 nm (sequencing channel 1, mNeonGreen and GFP), 546 nm (sequencing channel 2) and 638 nm (sequencing channel 3 and CellMask deep red). Emission filters used included Chroma ET450/50 (nuclear staining), Chroma ET525/50 (sequencing channel 1, mNeonGreen and GFP), 572/28 BrightLine HC (sequencing channel 2) and 680/42 BrightLine HC (sequencing channel 3, CellMask deep red). Exposure times were 90 ms for all channels except p65–mNeonGreen, ASC–GFP and C1C–EGFP, which were exposed for 150 ms. Objectives used were a Nikon ×10 CFI P-Apo, a Nikon ×20 CFI P-Apo or a Nikon ×40 CFI Apo ×40 WI with or without a ×1.5 tube lens inserted into the light path. A Hamamatsu Orca Flash4.0 LT+ camera was used in electronic shutter mode at full resolution (2,048 × 2,048). For a detailed step-by-step protocol, see Supplementary Protocol 2.
Image analysis of NIS-Seq data
Raw images of up to 14 NIS-Seq cycles were aligned by FFT-accelerated cross-correlation of nuclear staining images. Spots were detected by summing up all sequencing channels across the first three sequencing cycles, high-pass filtering, local maximum detection and brightness thresholding. Spot sequence information was aggregated across 5 × 5 pixels for every spot after high-pass filtering and eliminating negative values. Channel unmixing was performed by multiplying the channel vector of each cycle with the inverse matrix of average base-wise channel intensities. Non-G bases were called by the maximum of unmixed channels, whereas Gs were called at cycles with all unmixed intensities below 20% of the spot’s maximum unmixed intensity across all cycles. Sequences were assigned to the dictionary of known sequences (Brunello sgRNA sequences reverse complemented), allowing zero or one mismatch and no ambiguities. Dictionary-matched and dictionary-corrected spot sequences were assigned to nuclei, whose outlines were defined by Cellpose using the ‘nuclei’ model35, requiring the dominant sequence to make up more than two-thirds of total intensity of library spots in a given nucleus and the maximum signal intensity per nucleus across channels and sequencing cycles to pass a numeric threshold (7 × 105 for all cell types except 2 × 105 for iMacs). For a detailed step-by-step protocol, see Supplementary Protocol 3.
Image analysis of live phenotyping data
z-stacks were collapsed by averaging where applicable. Cell and nuclear outlines were defined by CellPose using the ‘cyto2’ and ‘nuclei’ models35. Nuclear translocation of mNeonGreen was quantified by calculating the Pearson correlation between nuclear staining and mNeonGreen fluorescence across pixels pertaining to each cell. GFP specking was quantified by local background subtraction (see below), high-pass filtering of fluorescent images, eliminating negative valued pixels and calculating the mean fluorescence across pixels pertaining to each cell before and after high-pass filtering. Cells with low mNeonGreen or GFP expression were excluded from downstream analysis. For local background subtraction, images were downsampled 8 × 8-fold. For each pixel in the full-resolution image, the local minimum across the 9 × 9 closest pixels in the downsampled image was subtracted, after which negative values were set to zero.
Analysis of HeLa genome-scale NIS-Seq perturbation screening data
Pairs of live phenotyping and NIS-Seq nuclear images acquired at corresponding stage positions were fine-mapped using FFT-accelerated cross-correlation. Phenotyping nuclei were assigned to the closest nucleus in shifted NIS-Seq data by centers of gravity, with a maximum movement distance of 11.1 µm and with the nuclear area matching within a two-fold margin. Any ambiguously mapping nuclei were excluded from further analysis.
Analysis of THP1 genome-scale NIS-Seq perturbation screening data
THP1 phenotypes were imaged using a ×10 objective with z-stacking to better cover small ASC specks across the cellular cytosol. Furthermore, live nuclear imaging turned out to be affected by the stimulation of cells. Therefore, the assignment of phenotype and NIS-Seq images described above for HeLa cells was modified. Instead of using live nuclear images, cell outlines derived using CellPose from z-aggregated membrane staining were shrunk by 5 pixels to predict the location of the nuclei. Potential pairs of phenotyping and NIS-Seq imaging fields of view were identified based on microscope stage positions. Images were scaled according to the relative magnification used and coarsely mapped at 8 × 8-downsampled resolution using FFT-accelerated cross-correlation. Best-correlated pairs of fields of view were fine-mapped at 2 × 2-downsampled resolution using FFT-accelerated cross-correlation. All subsequent analysis steps were performed as described above for HeLa cells.
Generation of single-gene perturbation cell lines for validation assays
sgRNAs were selected from the Toronto human knockout pooled library (TKOv3) and ordered as DNA oligonucleotides (Integrated DNA Technologies (IDT); Supplementary Table 1). Oligonucleotides were inserted into the CROPseq-iT7 lentiviral backbone using Golden Gate cloning. Purified and sequence-verified plasmids were used to produce lentivirus in HEK293T cells. Transduced cells were selected with puromycin for 3–5 d and screened for the phenotype of interest.
Primary human macrophage generation
Leukocyte-enriched buffy coats were mixed 1:1 with DPBS within 8 h after donation. Then, 15 ml of Ficoll-Paque Plus (Cytiva) was overlayed with 35 ml of blood–PBS mixture and centrifuged at 700 g for 20 min at 25 °C with disabled brakes. The upper serum-containing layer was removed, and the mononuclear cell layer was transferred to a fresh 50-ml reaction tube, filled up to 50 ml with DPBS and centrifuged at 340 g for 10 min at 25 °C. The supernatant was discarded, and the cells were resuspended in 50 ml of DPBS and pelleted for 5 min. Cells were resuspended in 500 µl of MACS buffer (DPBS, 2% FCS, 1 mM EDTA) and mixed with 100 µl of CD14 MicroBeads (Miltenyi). After incubation for 15 min at 4 °C, 25 ml of MACS buffer was added, and the cells were centrifuged at 340 g for 5 min. To separate CD14+ monocytes from the cell population, the cell pellet was gently resuspended in 3 ml of MACS buffer and added onto a LS column (Miltenyi Biotec) on a QuadroMACS Separator (Miltenyi Biotec). The column was washed three times with 3 ml of MACS buffer before eluting the cells in 3 ml of MACS buffer. After centrifuging at 340 g for 5 min, cells were recovered in 10 ml of complete media (RPMI GlutaMAX, 10% FCS, 1% sodium pyruvate, 1% penicillin–streptomycin) and counted. In total, 107 cells were incubated in 5 ml of complete media in the presence of 50 U ml−1 recombinant human macrophage colony-stimulating factor (rhM-CSF) (ImmunoTools) in six-well plates (Thermo Fisher Scientific) for 4 d.
Primary human macrophage harvesting
Differentiated macrophages were harvested by collecting the cells in suspension in a 50-ml reaction tube. Partially adherend cells were detached by adding 1 ml of DPBS containing 5 µM EDTA and incubation for 5 min at 37 °C. Both fractions were collected in a 50-ml reaction tube, counted and centrifuged at 340g for 5 min. In total, 2.5 × 106 cells were seeded in 2 ml of complete media containing 50 U ml−1 rhM-CSF in six-well plates (Greiner Bio-One) and incubated for 24 h.
Lentiviral production for primary human macrophage transduction
One day before transfection, 15 × 106 HEK293T cells were seeded in 30 ml of complete media (DMEM GlutaMAX, 10% FCS, 1% penicillin–streptomycin) in a 15-cm culture dish. Directly before transfection, the media were changed to 20 ml of 2% FCS-containing media. For transfection, 120 µl of Lipofectamine 2000 was incubated with 1,660 µl of Opti-MEM for 5 min and mixed with the following plasmids in a total volume of 1,780 µl of Opti-MEM: 7,040 ng of pCAGGS-VPx-VPr, 7,040 ng of lentiviral cargo plasmid, 10,560 ng of psPAX2 and 7,040 ng of pMD2.G. After 20 min, the transfection mix was added dropwise to the cells. The media were replaced after 4–6 h with complete media, and cells were incubated for 48 h at 37 °C and 5% CO2. The supernatant was filtered through a 0.45-µm syringe filter (VWR) and mixed with 1/3 volume of Lenti-X Concentrator (Takara). The mixture was incubated on ice for 45 min and centrifuged at 1,500 g for 45 min. The pellet was resuspended in 1/6 of the initial volume.
Primary human macrophage transduction
One day after harvest, the culture volume was reduced to 1 ml, and the macrophages were transduced with 400 µl of concentrated lentivirus in the presence of 10 µg ml−1 polybrene (Millipore). The media were changed after 6 h to 2 ml of complete media.
Sorting of EGFP+ primary human macrophages
Cells were harvested and adjusted to a concentration of 4 × 106 cells per ml−1 in complete media containing 20% FCS and 50 U ml−1 rhM-CSF. Cells were sorted using a Sony MA900 with a 130-µm sorting chip in sorting mode ‘yield’ into media containing 50% FCS and 50 U ml−1 rhM-CSF. Cells were pelleted, resuspended in complete media and seeded at a maximum density of 2 × 106 cells per milliliter in six-well plates.
Cas9 nucleofection of primary human macrophages
Harvested cells were washed in DPBS, counted and pelleted. In total, 2.5 × 106 cells were resuspended in 20 µl of P3 Primary Cell Nucleofector Solution (Lonza). Cells were nucleofected with 50 µg of SpCas9 v3 (IDT) in a 20-µl Nucleocuvette Strip (Lonza) using program CM-137. Cells were incubated in the strip for 5 min at 37 °C and subsequently recovered in warm complete media (50 U ml−1 rhM-CSF, no antibiotics). Cells were seeded into µ-Plate 96-squared-well plates (ibidi) at a density of 0.75 × 105 cells per milliliter. One day after nucleofection, the media were changed to complete media (RPMI GlutaMAX, 10% FCS, 1% sodium pyruvate, 1% penicillin–streptomycin, 50 U ml−1 rhM-CSF).
Inflammasome stimulation in primary human macrophages
Infected and nucleofected macrophages were primed with 20 ng ml−1 LPS (InvivoGen) for 3 h. To inhibit caspase-mediated cell death pathways, cells were incubated with 40 µM VX-765 (InvivoGen) and 50 µM Z-VAD-FMK (MCE, HY-16658B) for 30 min before stimulation. To activate an inflammasome, 1 µg ml−1 PA (BIOZOL, LBL-171E) together with 10 ng ml−1 of LFn-PrgI were added to the cells. After 1.5 h, cells were imaged in 75 µl of pre-warmed FluoroBrite DMEM (Gibco) in the presence of 1× CellMask DeepRed plasma membrane stain (Invitrogen) and 1 µg ml−1 Hoechst 33342. After imaging three z-stacks at a distance of 2.5 µm, cells were fixed using MeAA.
Preparation, transduction and sequencing of human epidermal sheets
Leftover skin tissue from clinical procedures was cut using a 6-mm biopsy punch (Kai Medical). Samples were incubated in 5 U ml−1 Dispase (Gibco, sterile filtered) for 2 h at 37 °C and washed once in PBS. Epidermal sheets were separated by gently pulling apart dermis and epidermis using sterile metal tissue tweezers. Epidermal sheets were kept in ex vivo culture media (DMEM GlutaMAX, 10% FCS, 1% penicillin–streptomycin, 25 µg ml−1 gentamycin, 1% amphotericin B) and infected with a concentrated CROPseq-iT7 lentiviral library (see above) in the presence of 10 µg ml−1 polybrene for 20 h at 37 °C. Epidermal sheets were washed twice in PBS and fixed in 4% PFA for 45 min at room temperature. Subsequently, sheets were washed three times in PBS, de-crosslinked in 0.5 M NaCl for 2 h at 65 °C and permeabilized in 70% ethanol for 30 min at room temperature. NIS-Seq was performed as described above with sheets floating in-solution in a 96-well plate (ibidi); imaging was performed by removing the liquid and centering the tissue in the well. Nuclei were stained after each cycle with 2 μM Hoechst 33342. F-actin was stained using 0.2 µM phalloidin-AF670.
Zero-knowledge image analysis of screening image data
To identify perturbations that lead to visual cellular changes, we performed zero-knowledge image analysis using deep learning. We used the established computer vision model SwinV2-T25, which was pre-trained on the ImageNet-1K dataset, modified it to embed monochromatic p65–mNeonGreen channel images of perturbed cells and removed the classification head. For each cell, we extracted the penultimate layer activations of the model, resulting in 768-dimensional vector embeddings. Using the k-means algorithm, the cells were divided into 200 clusters. Agglomerative clustering was applied to the centroids of those clusters to obtain 12 clusters. For illustration and visual inspection purposes, the 768-dimensional space of embeddings was projected to an auxiliary two-dimensional space using the UMAP algorithm.
Ethics statement
Buffy coats from healthy donors were obtained according to protocols accepted by the institutional review board at the University Hospital Bonn (local ethics vote Lfd. Nr. 075/14). Human skin biopsies were obtained from leftover skin of individuals undergoing plastic surgery at the University Hospital Bonn with informed consent of patients and according to protocols accepted by the institutional review board at the University Hospital Bonn (local ethics votes Lfd. Nr. 037/06, including amendments 2 and 3).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.