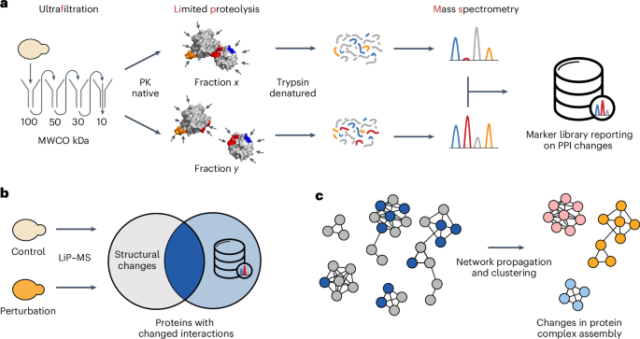
Cultivation of yeast cells
FLiP library
A 50-ml preculture of Saccharomyces cerevisiae (strain BY4716) was grown in a 250-ml Erlenmeyer flask from a single colony in yeast extract peptone dextrose at 30 °C with constant 150-rpm shaking for 6 h. The preculture was diluted 1:200 in 4 l of yeast extract peptone dextrose and equally distributed onto four 5-l Erlenmeyer flasks. The cultures were grown to an optical density at 600 nm of 0.8 ± 0.1.
HU stress
Four colonies of Saccharomyces cerevisiae (wild type BY4741 his3Δ1 ura3Δ0 leu2Δ0, met15Δ0; Gcn5 mutant BY4741 gcn5-E173A his3Δ200 ura3-52 leu2Δ0 lys2-801 ade2-101 trp1Δ63; Ada3-3xFLAG BY4741 MATa his3Δ1 leu2Δ0 ura3Δ0 met15Δ0 ada3-3xFLAG:kanMX6; Spt7-GFP BY4741 MATa his3∆1 leu2Δ0 ura3Δ0 met15Δ0 spt7-GFP:HIS3 from the yeast GFP collection113) were grown to an optical density at 600 nm of 0.8 ± 0.1 in synthetic complete 2% glucose (SC + glucose, CSM complete 40 ADE, Formedium DCS0039, Yest nitrogen base without amino acids, Formedium CYN0410, d-(+)-glucose, Sigma-Aldrich, cat. no. G7021) at 30 °C with constant 160-rpm shaking. For HU stress, at an optical density at 600 nm of 0.8 ± 0.1 the cells were transferred into 50 ml Falcon tubes (CELLSTAR, cat. no. 227261), spun down (1,000g, 5 min, 4 °C) and resuspended, washed once and resuspended in SC + 2% glucose medium supplemented with 200 mM HU (Sigma-Aldrich, cat. no. H8627). The cultures were incubated for 2 h at 30 °C with constant 160-rpm shaking.
Harvesting of yeast
FLiP library generation
The cultures were equally distributed onto eight 500-ml Corning polypropylene centrifuge tubes and spun down at 3,428g for 15 min at room temperature. Every batch was washed twice with 100 ml of PBS and spun down with the same parameters. Each pellet was dissolved in 5 ml of LiP buffer (20 mM HEPES, Sigma H4034, 150 mM KCl, Merck 1049360250 and 10 mM MgCl2, Sigma M2670 at pH 7.5, all components from Sigma-Aldrich) at room temperature and equally distributed into six 1.5 ml Eppendorf tubes. The cells were pelleted by centrifuging at 800g for 5 min at room temperature. The supernatant was discarded, and the cells were snap frozen in liquid nitrogen and stored at −80 °C.
HU stress
Here, 25 ml of each culture were collected in Falcon tubes (CELLSTAR, cat. no. 227261) and collected by centrifuging at 1,000g for 5 min at 4 °C. The pellets were washed with 10 ml PBS and centrifuged with the same parameters. The pellets were then resuspended in 1 ml of LiP buffer supplemented with 1× Roche Complete Protease Inhibitor EDTA-free (Sigma-Aldrich, cat. no. 11873580001) and transferred into 1.5-ml Eppendorf tubes. The cells were pelleted by centrifuging for 5 min at 800g at room temperature. The supernatant was discarded, and the cells snap frozen in liquid nitrogen and stored on ice before for processing.
Cell lysis
The yeast pellets were resuspended in LiP buffer with 1× Roche Complete Protease Inhibitor EDTA-free (Sigma-Aldrich, cat. no. 11873580001) and transferred into screwcap microcentrifuge tubes. A volume of glass beads equal to the volume of resuspended cells was added to each tube. The cells were lysed at 4 °C in a benchtop homogenizer (MP Biomedicals, Fastprep-24 5G) by eight cycles of 30-s beating at 5.5 Hz followed by a 200-s break. The microcentrifuge tubes were perforated with a hot needle on the bottom and the top of the tube. The tubes were positioned onto 1.5-ml Eppendorf tubes and spun at 800g for 30 s. The lysates were cleared of cell debris by centrifuging for 15 min at 21,500g at 4 °C. The supernatant was collected.
Serial ultrafiltration and RNase treatment
During serial ultrafiltration, we consecutively filtered the lysate through ultrafiltration devices with decreasing MWCOs. The retentates were collected, and the filtrates transferred onto the next highest MWCO filter. The process was repeated until the lowest MWCO filter was reached. Here we used 50-ml Amicon Ultra filters with MWCOs of 100, 50, 30 and 10 kDa (Sigma-Aldrich, cat. nos. UFC910008, UFC905008, UFC903008, UFC901008). All filters were washed with 5 ml of LiP buffer by centrifuging at 1,500g for 5 min immediately before loading the filters with lysate. All centrifugation steps were conducted at 4 °C. For good size separation, Amicon filters must be used sequentially, rather than using one or two consecutive cutoffs114,115.
Given the importance of RNA-binding proteins in the organization of cellular protein networks, we designed our FLiP library to capture changes in RNA-dependent protein–protein and protein–RNA-binding events, in addition to PPIs in general. We did this by diluting the lysate with LiP buffer with 1× Roche Complete Protease Inhibitor EDTA-free to 40 ml and adding 143 μl of RNase A (10 mg ml−1 stock solution in 10 mM sodium acetate, pH 7.4, Merck, R4875), RNase H (5,000 U ml−1, NEB, M0297), RNase I (10 U µl−1, Thermo Fisher Scientific, EN0601), RNase III (1 U µl−1, Thermo Fisher Scientific, AM2290) and RNase T1 (1,000 U µl−1, Thermo Fisher Scientific, EN0542). Samples were incubated at 4 °C on an analog tube roller for 1 h. These conditions were previously used to identify protein–RNA complexes confidently and at a proteome-wide scale by shifts in density gradient ultracentrifugation profiles between control and RNase treated samples116. Our rationale was that digestion of RNA will result in destabilization of protein–RNA complexes and RNA-dependent protein–protein complexes that can then be separated by serial ultrafiltration and will thus contribute FLiP markers. This requires partial digestion of RNA such that some of the protein–RNA complex remains intact and is retained in a high molecular weight fraction, whereas some of it disassembles and is in a lower molecular weight fraction. We assessed whether this is the case for ribosomal proteins and found that for 16 proteins of the 60S large ribosomal subunit (RPL10, RPL13A, RPL13B, RPL16B, RPL22A, RPL25, RPL28, RPL36B, RPL4A, RPL5, RPL6A, RPL6B, RPL7A, RPL7B, RPL8A, RPL8B) and for 18 proteins of the 40S small ribosomal subunit (RPS0B, RPS12, RPS14A, RPS15, RPS1A, RPS1B, RPS2, RPS20, RPS21A, RPS21B, RPS26A, RPS28A, RPS29A, RPS3, RPS31, RPS5, RPS7A, RPS7B), there was at least one FLiP marker between the 100-K and a lower molecular weight fraction. This suggests that the conditions we used enable the identification of FLiP markers of protein–RNA complexes or RNA-dependent protein–protein complexes.
After RNase treatment, four 50-ml Amicon Ultra filters with a MWCO of 100 kDa were loaded with 10 ml lysate, resulting in four replicates. Besides size fractionation, our goal during centrifugation was to concentrate the retained proteins while avoiding precipitation. For the different MWCO filters of 100, 50, 30 and 10 kDa. Retentate volumes of 1.5–2 ml, 200–300 μl, 200–300 μl and 100–300 μl, respectively, were achieved by centrifuging at 1,500g for 1 h, 30 min, 30 min and 1.5 h, respectively. For the 100 K fraction, we needed to ensure that the retentate did not become overconcentrated, which would lead to aggregation. To prevent aggregation, the volume was reduced to ~2 ml, requiring ~1 h of centrifugation. This is comparatively long because this fraction has a high initial protein concentration, which makes the filtration process slow. For the 50-K and 30-K fractions, the filtration process is much faster and a retention volume of ~200 μl can be reached within 30 min. For the 10-K fraction, the filtration process is again comparatively slow due to the small pore size. However, especially for this fraction, it is extremely important to concentrate the samples, since this is the fraction with the lowest total protein amount. Thus, we filtered for the 10-K fraction for 1 h 30 min to reach maximal concentration.
We added 83, 18, 3 and 3 μl of 50× Roche Complete Protease Inhibitor EDTA-free (Sigma-Aldrich, cat. no. 11873580001), respectively, to the retentates of the decreasing MWCO fractions to inhibit protease activity. We stored the retentates on ice in a 4 °C until further processing.
Protein concentration measurement
Protein concentrations of the lysate, as well as the fractions, were quantified with a bicinchoninic acid assay (Pierce BCA Protein Assay Kit, Thermo Fisher Scientific, ref. 23225).
SEC of different filter fractions
A single replicate of yeast lysate was fractionated by serial ultrafiltration as described above. Each fraction was filtered through a 2-ml Spin-X centrifuge tube filter (0.22 μm cellulose acetate) for 30 s with 800g at 4 °C. Concentration of each fraction was assessed by BCA and 100 μg of native proteins were injected to a SEC-s4000 column (300 × 7.8 mm, pore size 500 Å, particle size 5 μm, BioSep). Proteins were separated by SEC with LiP buffer at a flow rate of 500 μl min−1 for 45 min at 4 °C. Fraction collection was performed from 9 min to 33 min at 0.25 min per fraction. Only ultraviolet traces of eluting proteins were analyzed and are shown here, collected fractions were not further processed.
Native PAGE of protein assemblies in different filter fractions
A single replicate of yeast lysate was fractionated by serial ultrafiltration as described above and 100 μg of native proteins from each fraction were separated by size two different NuPAGE gels (NuPAGE Tris-Acetate Mini Protein Gels, 3 to 8%, Thermo EA0376BOX, NuPAGE Tris-Acetate Mini Protein Gels, 7%, Thermo EA0355BOX). Briefly, 7.5 μl sample was mixed with 2.5 μl 4× native PAGE sample buffer and separated on a NativePAGE gel (Thermo, BN2003) using the dark blue cathode buffer and applying 150 V for 60 min followed by 250 V for 30 min at 4 °C. Coomassie from the cathode buffer was fixed by microwaving the gel in fixing solution (40% methanol, Fisher Scientific 15631400, 10% acetic acid, Sigma-Aldrich 45754) for 45 s at ~1,000 W. The gel was shaken for 15 min at room temperature. For destaining, the gel was transferred into 100 ml 8% acetic acid solution and microwaved for 45 s at ~1,000 W and was kept in the destaining solution overnight. Gels were imaged on a Vilber Fusion FX with the Fusion FX6 Edge software using the ‘Coomassie optimal’ settings.
Sample randomization and blinding
The samples were randomized after adjusting concentration. The randomized order was kept for the rest of the protocol including MS data acquisition. The randomized samples were only labeled with numbers. Different investigators performed randomization and limited proteolysis as well as the tryptic digest to ensure blinding.
Limited proteolysis
The optimal protein concentration for LiP–MS is 2 mg ml−1 (ref. 30). However, to compare between filter fractions, we needed to adjust the protein concentrations of all samples to 1.3 mg ml−1, which is the lowest concentration across all filter fractions (found in the 10-K fraction). Limited proteolysis was performed as described previously31. Briefly, 50 μl of each sample were transferred into a PCR tube and heated to 25 °C for 5 min in a thermocycler (Biometra TRIO 48, 2070723). PK (Tritirachium album, 10 mg, Sigma-Aldrich, P2308) was added at an enzyme-to-substrate ratio of 1:100 (w/w) and incubated at 25 °C for 5 min. The digestion was stopped by heating the samples for 5 min at 99 °C. Tryptic control samples were generated in parallel except that 5 μl of water was added instead of PK. Samples were cooled on ice for 5 min. Proteins were denatured by adding a 10% (wt/vol) stock of sodium deoxycholate (DOC, Sigma 30970) to a final concentration of 5% (wt/vol) in a 2-ml 96-well plate.
Tryptic digest
Disulfide bonds were reduced by adding a stock of 200 mM tris-(2-carboxyethyl)-phosphine (TCEP, Sigma C4706) to a final concentration of 5 mM. The reaction was incubated at 37 °C for 40 min with 200-rpm shaking. The reduced disulfide bonds were alkylated by adding a freshly prepared stock of 1 M iodoacetamide (Sigma I1149) to a final concentration of 40 mM. The reaction was incubated at room temperature for 25 min without shaking and protected from light. The samples were diluted 1:5 with a 100 mM ammonium bicarbonate solution (Sigma-Aldrich A6141) to yield a DOC concentration of 1% (wt/vol). Finally, 1 μl of lysyl endopeptidase R (FUJIFILM Wako Pure Chemical Corporation, 129-02541) and 2 μl of trypsin (0.5 mg ml−1, Promega, V511C) were added. The samples were incubated at 37 °C overnight with 200-rpm shaking. The reaction was stopped the following day by adding 100% formic acid (Carl Roth GmbH) to a concentration of 3% (vol/vol) resulting in a pH below 2. DOC was removed by filtering the samples through a FiltrEX 96-well 0.2 μm polyvinyl difluoride (PVDF) membrane white filter plate (Corning, ref. 3508) by centrifuging at 2,000g for 2 min.
C18-cleanup and sample preparation
A 96-well C18 spin column plate (Nest Group, S8VL) was connected to a vacuum pump and was washed with 200 μl methanol (Fisher Scientific 15631400), followed by 100 μl buffer B (50% acetonitrile (ACN), Fisher Scientific A955-212, 0.1% formic acid, Carl Roth GmbH) and 3× 200 μl buffer A (5% ACN, Fisher Scientific A955-212, 0.1% formic acid, Carl Roth GmbH). Samples were loaded and washed with 3× 200 μl buffer A. Peptides were eluted with 3× 100 μl buffer B and dried in a vacuum centrifuge. All samples were resuspended in buffer A to a peptide concentration of 2 mg ml−1. iRT peptides (10×, Biognosys, Pp-2005) were added to the samples in a 1:30 dilution. For each combination of condition and/or fraction and sample type (LiP, tryptic control), a library sample consisting of an equal amount of peptide from every replicate was prepared.
Affinity-purification mass spectrometry
Yeast expressing ADA3-3xFLAG (BY4741 MATa his3Δ1 leu2Δ0 ura3Δ0 met15Δ0 ada3-3xFLAG:kanMX6) and wild-type control yeast cells (BY4741) were grown and gathered each in quadruplicates under control and HU stress as described above. Cell lysates were supplemented with 2.5 μl of benzonase (Sigma-Aldrich, E1014-5KU) and incubated under end over end rotation for 30 min at 4 °C. The postnuclear fraction was isolated by centrifugation at 21,000g, 4 °C, 30 min and the supernatant transferred to a new precooled Eppendorf tube. Protein concentrations were determined by Bradford assay and lysates were subsequently adjusted to a protein concentration of 2 mg ml−1 in lysis buffer. 0.8–1 mg lysate was used for affinity purification. To this end, samples were precleared with 30 μl of a 50% mouse IgG-agarose bead slurry (A0919, Sigma-Aldrich) for 60 min under end over end rotation at 4 °C. Beads were removed by centrifugation at 1,000g for 5 min. ADA3-3xFLAG was affinity purified from the precleared lysate by incubation with 25 μl of a 50% anti-FLAG-M2 bead slurry (A2220, Millipore) for 3 h under end to end rotation at 4 °C. ADA3-3xFLAG-depleted lysate was removed by centrifugation at 1,000g, 4 °C for 2 min and beads were washed twice with lysis buffer (without Roche protease inhibitor), followed by three washes with wash buffer (25 mM HEPES-KOH, Sigma H4034, pH 7.4, 100 mM KCl, Merck 1049360250, 2 mM MgCl2, Sigma M2670). Each washing step was performed by end over end rotation for 5 min at 4 °C, followed by centrifugation at 1,000g, 4 °C for 2 min. Purified material was eluted in 60 μl of 8 M urea (Sigma-Aldrich U5378), 100 mM ammonium bicarbonate (Sigma-Aldrich A6141) by shaking at 1,500 rpm and 37 °C on a thermomixer (Eppendorf) before centrifugation (1,000g, 25 °C, 2 min) and transfer of the supernatant to ProteinLoBind tubes. Samples were prepared for mass spectrometry by reduction with TCEP (Sigma C4706) and iodoacetamide-mediated alkylation (Sigma I1149), followed by dilution to 1 M urea with 100 mM ammonium bicarbonate (Sigma-Aldrich A6141). Each sample received 1 μg of trypsin and 1 μg of Lys-C and the digest was allowed to proceed at 37 °C for 20 h before acidification to pH 2 with formic acid. Samples were centrifuged at 21,000g for 20 min at 25 °C and desalted using Stage Tips (330 μg binding capacity) before resuspension in 15 μl of buffer A + iRT peptides (1:30).
LC–MS instrumentation
Samples were analyzed on an Orbitrap Fusion Lumos Tribrid mass spectrometer (Thermo Fisher) equipped with a nano-electrospray ion source and Waters Acquity M-Class UPLC system. Peptides were separated on a 40 cm × 0.75 μm i.d. column (New Objective, PF360-75-10-N-5) packed in house with 1.9-μm C18 beads (Dr. Maisch Reprosil-Pur 120). Liquid chromatography (LC) fractionation was achieved with the following gradients of buffer A (0.1% formic acid, Sigma-Aldrich 33015) and buffer B (99% ACN, Fisher Scientific A955-212, 0.1% formic acid, Sigma-Aldrich 33015): a linear gradient from 5 to 35% buffer B over 120 min, followed by 5 min with an isocratic constant concentration of 90% buffer B. The flow rate was 300 nl min−1, and the column was heated to 50 °C.
AP–MS samples were analyzed on an Orbitrap Eclipse Tribrid mass spectrometer (Thermo Fisher Scientific) equipped with a nano-electrospray ion source and a nano-flow LC system (Easy-nLC 1200, Thermo Fisher Scientific). Peptides were separated on a 40 cm × 0.75 mm i.d. column (New Objective, PF360-75-10-N-5) packed in house with 1.9-μm C18 beads (Dr. Maisch Reprosil-Pur 120). LC fractionation was achieved with the following gradients of buffer A (5% ACN, Fisher Scientific A955-212, 0.1% formic acid, Sigma-Aldrich 33015) and buffer B (95% ACN, Fisher Scientific A955-212, 0.1% formic acid, Carl Roth GmbH): a linear gradient from 3 to 30% buffer B over 120 min, followed by 5 min with an isocratic constant concentration of 90% buffer B. The flow rate was 300 nl min−1, and the column was heated to 50 °C.
DDA
For shotgun LC coupled with tandem MS (LC–MS/MS) DDA on the Orbitrap Fusion Lumos Tribrid mass spectrometer, 2 μl from each library sample were injected. MS1 spectra were acquired from 350 to 1,500 m/z at an orbitrap with a resolution of 120,000 with an automated gain control (AGC) target of 150% or 100-ms injection time. Precursors with an intensity exceeding 50,000 and a positively charged state between 2 and 5 were selected for data-dependent MS2 scans. Dynamic exclusion was applied after a single occurrence for 30 s with a 10-ppm mass tolerance. Selected precursors were isolated with a quadrupole and an isolation window of 1.2 m/z. Precursors were fragmented with high-energy collision-induced dissociation (HCD) with a fixed collision energy of 27%. MS2 spectra were acquired at an orbitrap resolution of 30,000, an automatically adapting scan range (minimum − precursor × charge + 10.0), and an AGC target of 200% or a dynamic injection time that automatically calculates the maximal time available. All MS data were collected as raw files using Xcalibur (v.4.2).
For shotgun LC–MS/MS DDA of the AP–MS data on the Orbitrap Eclipse Tribrid mass spectrometer, 4 μl from each sample were injected. MS1 spectra were acquired from 350 to 1,400 m/z at an orbitrap with a resolution of 120,000 with an AGC target of 200% or 54-ms injection time. Precursors with an intensity exceeding 50,000 and a positively charged state between 2 and 7 were selected for data-dependent MS2 scans. Dynamic exclusion was applied after a single occurrence for 20 s with a 10-ppm mass tolerance. Selected precursors were isolated with a quadrupole and an isolation window of 0.7 m/z. Precursors were fragmented with HCD with a fixed collision energy of 30%. MS2 spectra were acquired at an orbitrap resolution of 30,000, an automatically adapting scan range (minimum − precursor × charge + 10.0), and an AGC target of 200% or a maximum injection time of 54 ms. All MS data were collected as raw files using Xcalibur (v.4.3).
DIA
Aliquots of 2 μl of each sample were injected independently and measured in DIA mode. The DIA-MS method consisted of a survey MS1 scan from 350 to 2,000 m/z at a resolution of 120,000 with an AGC target of 50% or 100-ms injection time, followed by DIA in 41 variable-width isolation windows. The m/z isolation ranges are listed in Supplementary Table 10. Precursors were isolated by a quadrupole and fragmented with HCD with a collision energy of 28%. DIA-MS2 spectra were acquired with a scan range of 200 to 1,800 m/z at an orbitrap resolution of 30,000 with an AGC target of 200% or 54-ms injection time.
Data analysis
The code for the described data analysis pipelines is publicly available on GitHub117.
Search engines
The data of the serial ultrafiltration experiment generating the FLiP marker library was searched in Spectronaut v.15.6.211220.50606 and for all other experiments in Spectronaut v.17.1.221229.55965 (Biognosys). Hybrid libraries for the tryptic control and the LiP samples consisting of the corresponding DDA and DIA runs were created based on a Pulsar search using the default settings, with the exception of digest type, which was set to ‘semi-specific’ for the LiP samples only, and the minimal peptide length, which was set to six. The data were searched against the UniProt fasta database (strain S288c, UP000002311, July 2019). For the serial ultrafiltration experiment, the targeted data extraction was performed in Spectronaut 15 with default settings (BGS Factory Settings) except for the data filtering, which was set to ‘Qvalue percentile’ with a cutoff of 0.75. For all other experiments analyzed in Spectronaut 17, default settings were used. In all experiments, the FDR was set to 1% on precursor and protein levels and no values were imputed. The LiP and tryptic control samples were searched separately. From the LiP search, we exported peptide intensities, and from the tryptic control search, we exported protein intensities.
For the acetylation analysis, hybrid libraries for the tryptic control samples were generated with the same settings as above with an additional variable modification ‘Acetyl (K)’. The data was extracted using Spectronaut v.17.1.221229.55965 with default settings and imputation set to ‘Use Background Signal’. This ensures to include acetylated peptides that are present in only one condition.
The AP–MS data were searched in Spectromine v.3.2.22022.52329 (Biognosys). The search was performed with default parameters except for the proteotypicity filter, which was set to ‘Proteotypic only’ and normalization, which was not applied. The data were searched against the UniProt fasta database (strain S288c, UP000002311, July 2019) and a list of typical contaminants. The FDR was set to 1% on peptide and protein levels. For SAINT analysis, the control and HU-stressed samples were searched separately but together with their respective wild-type backgrounds and the quantification of proteins was exported based on number of spectral matches. For differential abundance analysis, control and HU-stressed samples and their respective backgrounds were searched together, and the quantification of proteins was exported as intensities.
Manual peak extraction
The acetylated peptides of Ada3 were manually extracted from the DIA files of the tryptic control using Skyline 64-bit (v.21.1). The MS/MS filtering was adjusted to the DIA method described above. All possible b- and y-ions up to a precursor charge minus one were extracted and manually filtered for coelution.
Data filtering
For all experiments, we required a peptide in the LiP dataset and its corresponding protein in the tryptic control dataset to be measured in at least biological triplicates in at least two conditions and/or fractions to be considered for further downstream analysis.
Data normalization
During limited proteolysis two types of peptide are formed. Fully tryptic peptides originate from trypsin cleavage alone, and semitryptic peptides result from PK cleavage followed by trypsin cleavage. We identified differences in accessibility between fractions with the following data analysis pipeline.
All peptide or protein intensities of a given LiP or tryptic control Spectronaut search were median normalized before exporting the data (global normalization). For the HU-induced stress response experiments, the peptide and/or protein intensities were median normalized before exporting the data. For the interface marker library, a separate Spectronaut search was conducted for every filter fraction and data were median normalized only within replicates of one fraction before exporting. The intensity values were based on Spectronaut normalized peak areas.
Further data processing was performed using the statistical software R (v.4.1.1) (R Foundation for Statistical Computing, http://www.R-project.org/). To account for differences in protein abundances across conditions, we corrected peptide intensities in condition c and replicate rep, \({I}_{{\mathrm{pep}},{c},{\mathrm{rep}}}\), by their corresponding protein intensities, \({I}_{{\mathrm{prot}},{c},{\mathrm{rep}}}\). For the interface marker library experiment, we further median normalized \({R}_{{\mathrm{pep}},{c},{\mathrm{rep}}}\) (equation 1), because the ratios significantly differed between filter fractions due to differences in protein abundances. This reflects that for most peptides, the ratio of peptide to protein stays constant across fractions and peptides for which this ratio changes across fractions pinpoint the location of a difference in accessibility between fractions and indicative of a PBI.
$${R}_{{\mathrm{pep}},c,{\mathrm{rep}}}=\,\frac{{I}_{{\mathrm{pep}},c,{\mathrm{rep}}}}{{I}_{{\mathrm{prot}},c,{\mathrm{rep}}}}$$
(1)
Significance analysis
To detect peptides for which \({R}_{{\mathrm{pep}},{c},{\mathrm{rep}}}\) is significantly different between conditions (or filter fractions), we perform a one-way ANOVA test for all peptides detected in two or more conditions. We calculate the mean intensity of the peptide pep in condition c as:
$${\mathrm{Mean}}_{\mathrm{pep},c}=\frac{{\sum }_{{\mathrm{rep}}=1}^{{n}_{{\mathrm{pep}},c}}\,{R}_{{\mathrm{pep}},c,{\mathrm{rep}}}}{{n}_{{\mathrm{pep}},c}}$$
(2)
where \({n}_{\mathrm{pep},{c}}\) is the number of biological replicates (three or four) that a peptide was detected in condition c. Next, we propagate the error that results from the joint uncertainty in the peptide intensity and the protein intensity for every \({\mathrm{Mean}}_{\mathrm{pep},{c}}\) as:
$${\mathrm{s.d.}}_{\mathrm{pep},c}={\mathrm{Mean}}_{\mathrm{pep},c}\,\sqrt{{\left(\frac{{\rm{\sigma }}({I}_{{\mathrm{pep}},c})}{{\rm{\mu }}({I}_{{\mathrm{pep}},c})}\right)}^{2}+{\left(\frac{{\rm{\sigma }}({I}_{{\mathrm{prot}},c})}{{\rm{\mu }}({I}_{{\mathrm{prot}},c})}\right)}^{2}\,}$$
(3)
where σ denotes the standard deviation and μ the mean of the peptide pep and the corresponding protein prot intensities in condition c. The between group mean squared error is given by:
$${\mathrm{MS}}_{{\mathrm{between}},{\mathrm{pep}}}=\,\frac{\sum _{c}{n}_{{\mathrm{pep}},c}{\left({\mathrm{Mean}}_{{\mathrm{pep}},c}-\frac{\sum _{c}{\mathrm{Mean}}_{{\mathrm{pep}},c}}{{N}_{\mathrm{pep}}}\right)}^{2}\,}{{C}_{\mathrm{pep}}}$$
(4)
where Npep is the number of times a peptide was measured across all conditions and Cpep the number of conditions it was detected in. The within group mean squared error is given by:
$${\mathrm{MS}}_{{\mathrm{within}},{\mathrm{pep}}}=\,\frac{\sum _{\mathrm{frac}}({n}_{{\mathrm{pep}},c}-1\,)\,{{\mathrm{s.d.}}_{{\mathrm{pep}},c}}^{2}\,}{{N}_{\mathrm{pep}}-\,{C}_{\mathrm{pep}}}$$
(5)
Finally, the F value is given by:
$${F}_{\mathrm{pep}}=\,\frac{{\mathrm{MS}}_{{\mathrm{between}},{\mathrm{pep}}}}{{\mathrm{MS}}_{{\mathrm{within}},{\mathrm{pep}}}}$$
(6)
The F value Fpep follows an F distribution with (\({C}_{\mathrm{pep}}-1\), \({N}_{\mathrm{pep}}-\,{C}_{\mathrm{pep}}\)) degrees of freedom. The P value is given by the probability of observing an event at least as extreme as the Fpep value. The P values are corrected for multiple testing with the Benjamini–Hochberg method and reported as q values118.
Multiple peptides can overlap because they map to the same protein regions because they originate from the same fully tryptic parent peptide. This can be caused by distinct PK cleavage sites since PK cleavage is a stochastic process or by missed cleavages. We group those peptides back to the longest identified fully tryptic parent and group the q value by its median over all peptides. We name those uniquely identified positions as FLiP markers.
Marker classification
In the FLiP marker library, some proteins elute in lower molecular weight fractions than expected by their size. This can be due to the property of the protein itself or by cleavage events occurring in the cell or during the experimental procedure. Due to the nonstrict nature of the filter cutoffs, we classify proteins as eluting in the correct fraction if half of their molecular weight does not exceed the filter cutoff (for example an 80-kDa protein is allowed to elute in the 30-kDa fraction where all proteins are expected to be below 50 kDa, but not in the 10-kDa fraction where all proteins are expected to be below 30 kDa).
Proteins ending up in the wrong fraction can lead to significant changes detected by FLiP. We perform a Tukey honest significant difference test with adjustment for multiple testing (Benjamini–Hochberg) to assess the significance of a change between any two fractions. If a change is only significant when including the wrong molecular weight fraction, we classify the marker as low confident, high confident otherwise.
Also, we add information about previous evidence for a marker to be located at a known binding interface by annotating whether the marker peptide is overlapping with an InterPro domain annotated with the GO-terms ‘protein binding’ or ‘RNA binding’ or if its average distance to a binding interface in a PDB structure is <2.6 Å (see the section ‘Distance of FLiP markers to PBIs of multimeric structures’).
Integrating FLiP marker library into LiP–MS datasets
A peptide detected in any LiP–MS dataset was classified as FLiP–MS marker peptide if it was overlapping by at least 50% with a peptide from the library.
Domain-based GO-enrichment analysis
Protein domain GO-annotation on molecular function for all detected peptides was downloaded from the InterPro database35 (December 2021). A Fisher Exact test was computed to detect domains enriched in the FLiP library compared to all detected peptides.
Disorder analysis
Protein disorder information for the yeast proteome was downloaded from UniProt119 (July 2022). If a peptide of the FLiP dataset was at least partially overlapping with a disordered region it was classified as disordered. The percentage of disordered peptides for all nonsignificant and significant peptides of the FLiP dataset was calculated. A Fisher Exact test was performed to test against the null hypothesis that significant FLiP peptides are not disordered.
Distance of FLiP markers to PBIs of multimeric structures
We collected experimental structures associated with the relevant UniProt accession numbers119 as defined by SIFTS120 on 9 May 2022, through the SWISS-Model Repository API121. Analysis was performed on oligomers depicted as ‘biological assembly 1’. In case of multiple oligomers for the same UniProt accession number, we chose the structure with the most subunits (biggest), a structure at random (random) or the structure that best reflected the experimental data was considered. This left between 364 and 394 oligomeric structures for analysis depending on the selection method (Supplementary Table 2). Interface residues were identified by computing relative solvent accessibilities as described by Lee and Richards122 as implemented in OpenStructure123. Residues were defined as ‘interface’ if the change was above 25% when computed on the full oligomers as compared to single chains in isolation.
All FLiP peptides of a given protein were mapped to the corresponding PDB structure by sequence. FLiP peptides absent from the structure were discarded from the analysis. For all remaining peptides, distances (Cα carbon to Cα carbon) to these interface residues determined the labeling of the FLiP peptides. Each peptide residue was assigned the minimum average distance to any of the interface residues. If the distance was below the selected cutoff (2.6 or 0.3 Å), the peptide was labeled as interface associated (that is, positive). Whenever an oligomer contained several occurrences of the same peptide, a peptide was randomly selected for the labeling procedure. A ROC area under the curve (AUC) was used to determine whether the significance level of a peptide predicted the proximity to an oligomer interface. For the ROC analysis we classified marker peptides located at interfaces as true positives, marker peptides not located at interfaces as false positives, nonchanging peptides located at interfaces as false negatives and nonchanging peptides not located at interfaces as true negatives, resulting in AUCs of 0.54 (biggest), 0.54 (random) and 0.61 (best) when using 2.6 Å as a cutoff, and AUCs of 0.61 (biggest), 0.63 (random), 0.71 (best) at the more stringent 0.3-Å cutoff. The analysis was done in python v.3.7 and packages pickle5 v.0.0.11, matplotlib v.3.1.3 and sklearn v.0.22.1.
AlphaFold3 predictions of heterodimeric protein complexes
We used network analysis (sections below) to extract heterodimeric protein complexes from the Complex Portal database, requiring that they had FLiP markers for both subunits and no previous structural characterization. Around 30 heterodimeric protein structures were predicted on the AlphaFold server (https://alphafoldserver.com/about) with seed 42, of which only two had a low predicted alignment error and are reported in this study.
Calculating the distance of peptides to the protein surface
If FLiP markers are enriched in PBIs, they should be located closer to the protein surface compared to other LiP hits, since the latter can contain buried small-molecule binding sites. To test this hypothesis, we first defined the surface by the MSMS algorithm124 (MSMS v.2.5.7, get_surface, Bio.PDB.ResidueDepth v.1.83) with increasing probe radius. Increasing the radius will define buried sites as not located at the surface. The distance of a peptide to the surface was calculated with scipy.spatial.cKDTree (v.1.13.0) from the PK cleavage site for semitryptic and from the trypsin cleavage site for fully tryptic peptides.
Acetylation analysis
For each comparison (wild-type control to wild-type HU, mutant control to mutant HU, wild-type control to mutant control, wild-type HU to mutant HU), a separate Spectronaut search with the respective sample files was performed with the spectral library containing acetylated peptides and imputation ‘Use Background Signal’. Each dataset was median normalized in Spectronaut (global normalization) before exporting the peptide and protein intensities separately. Differential abundance analysis was performed as described above.
AP–MS analysis
High confidence interactors of Ada3 under control and HU-stressed conditions were determined separately by comparing the spectral counts of the FLAG-tagged to the nontagged samples in SAINT125. We identified 238 interactors of Ada3-FLAG by requiring a SAINT probability of 1 and that the protein was detected in four out of four replicates in the pull-down with a minimal average spectral count of 4.5. Intensities of high confidence interactors (SAINT = 1) for FLAG-tagged and nontagged samples under control and HU stress were normalized together by total area sums and only proteins detected in at least triplicates per condition were considered for downstream analysis. The ratios of prey to bait \({R}_{{\mathrm{prey}},{\mathrm{bait}},{\mathrm{treatment}}}\) was defined as follows, where \({{\mu }}(I)\) denotes the mean protein intensity of the respective protein in either the FLAG-tagged or nontagged cells under untreated or HU-treated conditions. In our case Ada3 is the bait.
$${R}_{{\mathrm{prey}},{\mathrm{bait}},{\mathrm{treatment}}}=\,\frac{\frac{{{\mu }}({I}_{{\mathrm{prey}},{\mathrm{FLAG}},{\mathrm{treatment}}})}{{{\mu }}({I}_{{\mathrm{prey}},{\mathrm{non}}-{\mathrm{FLAG}},{\mathrm{treatment}}})}}{\frac{{{\mu }}({I}_{{\mathrm{bait}},{\mathrm{FLAG}},{\mathrm{treatment}}})}{{{\mu }}({I}_{{\mathrm{bait}},{\mathrm{non}}-{\mathrm{FLAG}},{\mathrm{treatment}}})}}$$
(7)
We propagated the error of each ratio as:
$$\begin{array}{l}{\mathrm{s.d.}}_{{\mathrm{prey}},{\mathrm{bait}},{\mathrm{treatment}}}\\={R}_{{\mathrm{prey}},{\mathrm{bait}},{\mathrm{treatment}}}\,\sqrt{\begin{array}{c}{\left(\displaystyle\frac{{\rm{\sigma }}\left({I}_{{\mathrm{prey}},{\mathrm{FLAG}},{\mathrm{treatment}}}\right)}{{\rm{\mu }}\left({I}_{{\mathrm{prey}},{\mathrm{FLAG}},{\mathrm{treatment}}}\right)}\right)}^{2}+{\left(\displaystyle\frac{{\rm{\sigma }}\left({I}_{{\mathrm{prey}},{\mathrm{non}}-{\mathrm{FLAG}},{\mathrm{treatment}}}\right)}{{\rm{\mu }}\left({I}_{{\mathrm{prey}},{\mathrm{non}}-{\mathrm{FLAG}},{\mathrm{treatment}}}\right)}\right)}^{2}\,\\ +{\left(\displaystyle\frac{{\rm{\sigma }}({I}_{{\mathrm{bait}},{\mathrm{FLAG}},{\mathrm{treatment}}})}{{\rm{\mu }}({I}_{{\mathrm{bait}},{\mathrm{FLAG}},{\mathrm{treatment}}})}\right)}^{2}+{\left(\displaystyle\frac{{\rm{\sigma }}({I}_{{\mathrm{bait}},{\mathrm{non}}-{\mathrm{FLAG}},{\mathrm{treatment}}})}{{\rm{\mu }}({I}_{{\mathrm{bait}},{\mathrm{non}}-{\mathrm{FLAG}},{\mathrm{treatment}}})}\right)}^{2}\end{array}}\end{array}$$
(8)
where σ denotes the standard deviation and μ the mean of the given protein intensity. We calculate the t-statistic as:
$${t}_{{\mathrm{prey}}}=\frac{{R}_{{\mathrm{prey}},{\mathrm{bait}},{\mathrm{HU}}-{\mathrm{treated}}}-{R}_{{\mathrm{prey}},{\mathrm{bait}},{\mathrm{untreated}}}}{\sqrt{\frac{{{\mathrm{s.d.}}_{{\mathrm{prey}},{\mathrm{bait}},{\mathrm{HU}}-{\mathrm{treated}}}}^{2}+{{\mathrm{s.d.}}_{{\mathrm{prey}},{\mathrm{bait}},{\mathrm{untreated}}}}^{2}}{3}}\,}$$
(9)
The t-statistic follows a Student’s t distribution with four degrees of freedom. The P value is given by the probability of observing an event at least as extreme as the t value P(T > tprey). The log2 fold change is given by the ratio log2(Rprey,bait,HU-treated/Rprey,bait,untreated).
Network analysis
All 586 proteins with at least one significantly changing FLiP marker in the HU-induced DNA replication stress screen were projected on a PPI network based on Complex Portal40 (16 March 2021). Out of those 586 proteins, only 206 proteins are part of the network. Note that we used this database as ground truth because complexes are defined with high stringency, based on experimental physical interaction data, but in principle another database could also be used. The network was analyzed in R (v.4.1.1) using igraph (v.1.2.6). The network was filtered for self-loops and redundancies. All edges were considered undirected and unweighted. First, the network was propagated from the proteins with interface marker hits in the HU stress screen using the PageRank algorithms with a dampening factor of 0.9 (ref. 126). The most important part of the network was selected based on scoring in the top 40% of this propagation resulting in a network consisting of 722 proteins. We chose the dampening factor of 0.9 and the 40% PageRank score cutoff as it yielded a network that was neither too sparse nor too dense, making it possible to identify protein complexes. We then used the walktrap algorithm127 with four steps to cluster the resulting network, again choosing the number of steps to obtain good separation between clusters. Finally, we selected clusters and/or complexes with at least four subunits, aiming to filter out dimeric complexes that are typically less well annotated. This is the final network that we report, containing 607 proteins with 56 complexes. Note that that there is no single optimal threshold for any of these steps, and a user may choose them differently depending on their systems and the goal of the experiment. We named the resulting clusters based on the protein complex from the Complex Portal database with the highest number of changing FLiP markers present in that cluster, reasoning that the higher the number of marker changes in a given protein complex, the more likely it is that this specific complex is rearranging. Because many protein complexes share subunits, we sometimes provide more than one name for a cluster if all changing markers are part of multiple complexes (for example, DNA-directed RNA polymerase I, II or III complexes or the INO80–Swr1–Nu4A complex). For the comparison of the response in PPI changes of wild type and the Gcn5 catalytic dead cells, the data were reduced to only contain protein regions detected in both datasets.
Data visualization
Data were visualized using the statistical software R (v.4.1.1) with the packages ggplot2 (v.3.3.5), RColorBrewer (v.1.1.2), ComplexHeatmap (v.2.8.0), igraph (v.1.2.6) and EnhancedVolcano (v.1.18.0). Peak groups and protein sequence localization plots were visualized using Microsoft Excel (v.16.0.5332.1000).
Protein structure visualization
Protein structures were retrieved from the PDB128 and visualized in PyMol (v.2.4.1). The peptide sequences were aligned to the PDB sequence and colored according to their classification.
SDS–PAGE and immunoblotting of C-terminally GFP-tagged Spt7
BY4741 MATa his3Δ1 leu2Δ0 ura3Δ0 met15Δ0 spt7-GFP:HIS3 from the yeast GFP collection113 was grown and harvested in a single replicate under control and HU stress as described above. Protein concentration was adjusted to 3 mg ml−1 and 40 μl were mixed with 360 μl 5% (w/v) sucrose stock (5% (w/v) sucrose, 150 mM NaCl, 1 mM MgCl2, 100 mM HEPES pH 7.4, 2× Roche Complete protease inhibitor). Proteins were precipitated on ice for 1 h by adding trichloroacetic acid to a final concentration of 9% (v/v), followed by centrifugation at 21,000g for 10 min at 4 °C. The supernatant was discarded, and the protein pellets washed twice with ice-cold acetone (500 μl per sample, spin in between washes at 21,000g for 10 min at 4 °C). Pellets were air-dried for 5–10 min after removing residual acetone and resuspended in LDS NuPage Sample buffer (NP0007, Thermo Fisher) containing 137.5 mM DTT and 312.5 mM Tris base. Samples were heated at 95 °C for 10 min under constant agitation (Eppendorf thermomixer, 1,500 rpm) before separation on NuPage 4–12% Bis/Tris gradient gels. Proteins were then transferred to a PVDF membrane and membranes blocked with 5% milk powder (w/v) in Tris-buffer saline (TBS) + 0.1% Tween for 1 h before incubation with an anti-GFP antibody (Roche, 11814460001, 1:1,000 in 2% BSA/TBS + 0.1% Tween) under constant agitation for 16 h at 4 °C. Membranes were rinsed with TBS + 0.1% Tween 3× 5 min and incubated with HRP-conjugated secondary antibody (Invitrogen, A16078, 1:10,000 in 5% milk powder (w/v) in TBS + 0.1% Tween) for 1 h and rinsed with TBS + 0.1% Tween 3× 5 min before application of ECL substrate (ECL Clarity) and image acquisition (Vilber Fusion FX).
Spot growth assay
Yeast cells were grown in liquid culture for >16 hours to an optical density (OD600) between 0.6 0.8. These cultures were diluted to a starting OD600 of 0.1, then fivefold serial dilutions were spotted onto different media in 2-µl amounts and grown at 30 °C for 4 days. Plates comprised either: synthetic complete medium and 2% glucose, or synthetic complete medium, 2% glucose and 100 mM HU (Sigma-Aldrich). Images were acquired on a Bio-Rad Gel Doc XL+ (Bio-Rad) using transmitted white light.
Fluorescence microscopy
Cells expressing Dcp2-GFP from the endogenous locus were imaged using a Nikon Ti-E Eclipse (Nikon Instruments), controlled by Micro-Manager v.1.4.23 software129, with a Plan Apo ×60 1.4 NA objective. A CoolLED pE-300 (CoolLED) light source was used for fluorescence illumination. To rapidly assess P-body formation in different conditions, a 1-ml sample of cells was concentrated by centrifugation (600g, 2 min) and visualized immediately by wet mount.
Time-lapse microscopy and analysis
Cells were grown for >16 h to OD600 = 0.6–0.8, diluted to OD600 = 0.2, then loaded onto a CellASIC ONIX Y04 microfluidic plate (Merck Millipore), which was mounted on the microscope described above, housed in a temperature-controlled incubator set to 30 °C. Cells were grown under normal conditions (synthetic complete medium, 2% glucose) for 3 h, switched to the stress condition (synthetic complete medium, 2% glucose, 200 mM HU) for 3 h, then switched back to normal conditions for 6 h. Media switching was controlled using CellASIC ONIX software. The flow rate was maintained at 3.5 psi throughout. Brightfield images were acquired every 15 min and fluorescence images every 30 min, in a single focal plane. Images were acquired more rapidly (5-min intervals for both channels), for a 40-min period centered on the switch to stress conditions. Time-lapse videos were analyzed by extracting the mean and standard deviation of fluorescence pixel intensities within each cell at each timepoint, based on masks generated by segmenting cells in the brightfield channel, using YeaZ software130. The averaged single-cell quantifications of Dcp2-mNG dispersion index were calculated as the variance in pixel intensities within a cell divided by mean pixel intensity within the same cell. For image display, 16-bit raw microscopy images were background subtracted in Fiji using a 200 pixel rolling ball radius. They were linearly scaled in Fiji, converted to 8-bit, and displayed in greyscale. In each figure, scaling was identical between images representing different time points and/or conditions, to allow accurate comparison of the signals. All quantifications were carried out on 16-bit images after background subtraction (200-pixel rolling ball radius).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.